The fusion glycoprotein of human respiratory syncytial virus facilitates virus attachment and infectivity via an interaction with cellular heparan sulfate
- PMID: 10864656
- PMCID: PMC112152
- DOI: 10.1128/jvi.74.14.6442-6447.2000
The fusion glycoprotein of human respiratory syncytial virus facilitates virus attachment and infectivity via an interaction with cellular heparan sulfate
Abstract
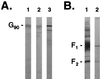
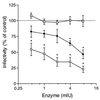
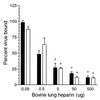
Human respiratory syncytial virus (RSV) F glycoprotein (RSV-F) can independently interact with immobilized heparin and facilitate both attachment to and infection of cells via an interaction with cellular heparan sulfate. RSV-glycosaminoglycan (GAG) interactions were evaluated using heparin-agarose affinity chromatography. RSV-F from A2- and B1/cp-52 (cp-52)-infected cell lysates, RSV-F derived from a recombinant vaccinia virus, and affinity-purified F protein all bound to and were specifically eluted from heparin columns. In infectivity inhibition studies, soluble GAGs decreased the infectivity of RSV A2 and cp-52, with bovine lung heparin exhibiting the highest specific activity against both A2 (50% effective dose [ED(50)] = 0.28 +/- 0.11 microg/ml) and cp-52 (ED(50) = 0.55 +/- 0. 14 microg/ml). Furthermore, enzymatic digestion of cell surface GAGs by heparin lyase I and heparin lyase III but not chondroitinase ABC resulted in a significant reduction in cp-52 infectivity. Moreover, bovine lung heparin inhibited radiolabeled A2 and cp-52 virus binding up to 90%. Taken together, these data suggest that RSV-F independently interacts with heparin/heparan sulfate and this type of interaction facilitates virus attachment and infectivity.
Figures


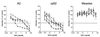
Similar articles
-
Respiratory syncytial virus with the fusion protein as its only viral glycoprotein is less dependent on cellular glycosaminoglycans for attachment than complete virus.Virology. 2002 Mar 15;294(2):296-304. doi: 10.1006/viro.2001.1340. Virology. 2002. PMID: 12009871
-
Heparin-dependent attachment of respiratory syncytial virus (RSV) to host cells.Arch Virol. 1997;142(6):1247-54. doi: 10.1007/s007050050156. Arch Virol. 1997. PMID: 9229012
-
Identification of linear heparin-binding peptides derived from human respiratory syncytial virus fusion glycoprotein that inhibit infectivity.J Virol. 2007 Jan;81(1):261-71. doi: 10.1128/JVI.01226-06. Epub 2006 Oct 18. J Virol. 2007. PMID: 17050595 Free PMC article.
-
Respiratory syncytial virus entry mechanism in host cells: A general overview.Mol Microbiol. 2023 Sep;120(3):341-350. doi: 10.1111/mmi.15133. Epub 2023 Aug 3. Mol Microbiol. 2023. PMID: 37537859 Review.
-
Heparan sulfate glycosaminoglycans as primary cell surface receptors for herpes simplex virus.Adv Exp Med Biol. 1992;313:341-53. doi: 10.1007/978-1-4899-2444-5_33. Adv Exp Med Biol. 1992. PMID: 1332443 Review.
Cited by
-
Actin cytoskeleton remodeling disrupts physical barriers to infection and presents entry receptors to respiratory syncytial virus.J Gen Virol. 2023 Nov;104(11):001923. doi: 10.1099/jgv.0.001923. J Gen Virol. 2023. PMID: 38015055 Free PMC article.
-
Elevated temperature triggers human respiratory syncytial virus F protein six-helix bundle formation.Virology. 2010 Jan 20;396(2):226-37. doi: 10.1016/j.virol.2009.10.040. Epub 2009 Nov 18. Virology. 2010. PMID: 19922971 Free PMC article.
-
N-glycans of F protein differentially affect fusion activity of human respiratory syncytial virus.J Virol. 2001 May;75(10):4744-51. doi: 10.1128/JVI.75.10.4744-4751.2001. J Virol. 2001. PMID: 11312346 Free PMC article.
-
Interaction of Nucleolin with the Fusion Protein of Avian Metapneumovirus Subgroup C Contributes to Viral Replication.Viruses. 2022 Jun 27;14(7):1402. doi: 10.3390/v14071402. Viruses. 2022. PMID: 35891383 Free PMC article.
-
Identification of deletion mutant respiratory syncytial virus strains lacking most of the G protein in immunocompromised children with pneumonia in South Africa.J Virol. 2011 Aug;85(16):8453-7. doi: 10.1128/JVI.00674-11. Epub 2011 Jun 15. J Virol. 2011. PMID: 21680500 Free PMC article.
References
-
- Anderson K, Stott E J, Wertz G W. Intracellular processing of the human respiratory syncytial virus fusion glycoprotein: amino acid substitutions affecting folding, transport and cleavage. J Gen Virol. 1992;73:1177–1188. - PubMed
-
- Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248. - PubMed
-
- Cardin A D, Weintraub H J R. Molecular modeling of protein-glycosaminoglycan interactions. Arteriosclerosis. 1989;9:21–32. - PubMed
-
- Chen Y, Maguire T, Hileman R E, Fromm J R, Esko J D, Linhardt R J, Marks R M. Dengue virus infectivity depends on envelope protein binding to target cell heparan sulfate. Nat Med. 1997;3:866–871. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

