Kaposi's sarcoma-associated herpesvirus open reading frame 50/Rta protein activates the entire viral lytic cycle in the HH-B2 primary effusion lymphoma cell line
- PMID: 10846108
- PMCID: PMC112123
- DOI: 10.1128/jvi.74.13.6207-6212.2000
Kaposi's sarcoma-associated herpesvirus open reading frame 50/Rta protein activates the entire viral lytic cycle in the HH-B2 primary effusion lymphoma cell line
Abstract
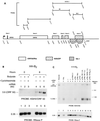
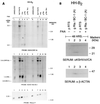
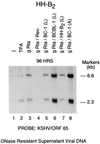
Rta, the gene product of Kaposi's sarcoma-associated herpesvirus (KSHV) encoded mainly in open reading frame 50 (ORF50), is capable of activating expression of viral lytic cycle genes. What was not demonstrated in previous studies was whether KSHV Rta was competent to initiate the entire viral lytic life cycle including lytic viral DNA replication, late-gene expression with appropriate kinetics, and virus release. In HH-B2, a newly established primary effusion lymphoma (PEL) cell line, KSHV ORF50 behaved as an immediate-early gene and autostimulated its own expression. Expression of late genes, ORF65, and K8.1 induced by KSHV Rta was eliminated by phosphonoacetic acid, an inhibitor of viral DNA polymerase. Transfection of KSHV Rta increased the production of encapsidated DNase-resistant viral DNA from HH-B2 cells. Thus, introduction of an ORF50 expression plasmid is sufficient to drive the lytic cycle to completion in cultured PEL cells.
Figures
Similar articles
-
CCAAT/enhancer-binding protein-alpha is induced during the early stages of Kaposi's sarcoma-associated herpesvirus (KSHV) lytic cycle reactivation and together with the KSHV replication and transcription activator (RTA) cooperatively stimulates the viral RTA, MTA, and PAN promoters.J Virol. 2003 Sep;77(17):9590-612. doi: 10.1128/jvi.77.17.9590-9612.2003. J Virol. 2003. PMID: 12915572 Free PMC article.
-
Identification of Novel Kaposi's Sarcoma-Associated Herpesvirus Orf50 Transcripts: Discovery of New RTA Isoforms with Variable Transactivation Potential.J Virol. 2016 Dec 16;91(1):e01434-16. doi: 10.1128/JVI.01434-16. Print 2017 Jan 1. J Virol. 2016. PMID: 27795414 Free PMC article.
-
ARID3B: a Novel Regulator of the Kaposi's Sarcoma-Associated Herpesvirus Lytic Cycle.J Virol. 2016 Sep 29;90(20):9543-55. doi: 10.1128/JVI.03262-15. Print 2016 Oct 15. J Virol. 2016. PMID: 27512077 Free PMC article.
-
Lytic cycle switches of oncogenic human gammaherpesviruses.Adv Cancer Res. 2007;97:81-109. doi: 10.1016/S0065-230X(06)97004-3. Adv Cancer Res. 2007. PMID: 17419942 Review.
-
PAN RNA: transcriptional exhaust from a viral engine.J Biomed Sci. 2020 Mar 7;27(1):41. doi: 10.1186/s12929-020-00637-y. J Biomed Sci. 2020. PMID: 32143650 Free PMC article. Review.
Cited by
-
A Kaposi's sarcoma-associated herpesvirus/human herpesvirus 8 ORF50 deletion mutant is defective for reactivation of latent virus and DNA replication.J Virol. 2005 Mar;79(6):3479-87. doi: 10.1128/JVI.79.6.3479-3487.2005. J Virol. 2005. PMID: 15731242 Free PMC article.
-
Direct interactions of Kaposi's sarcoma-associated herpesvirus/human herpesvirus 8 ORF50/Rta protein with the cellular protein octamer-1 and DNA are critical for specifying transactivation of a delayed-early promoter and stimulating viral reactivation.J Virol. 2007 Aug;81(16):8451-67. doi: 10.1128/JVI.00265-07. Epub 2007 May 30. J Virol. 2007. PMID: 17537858 Free PMC article.
-
The Herpesvirus saimiri replication and transcription activator acts synergistically with CCAAT enhancer binding protein alpha to activate the DNA polymerase promoter.J Virol. 2005 Nov;79(21):13548-60. doi: 10.1128/JVI.79.21.13548-13560.2005. J Virol. 2005. PMID: 16227275 Free PMC article.
-
Number of and distance between response elements in Kaposi's sarcoma-associated herpesvirus ORF57 promoter influence its activation by replication and transcription activator and its repression by interferon regulatory factor 7.Arch Virol. 2010 Mar;155(3):361-6. doi: 10.1007/s00705-009-0576-5. Epub 2009 Dec 29. Arch Virol. 2010. PMID: 20039088 Free PMC article.
-
Kaposi's sarcoma-associated herpesvirus/human herpesvirus 8 ORF50/Rta lytic switch protein functions as a tetramer.J Virol. 2007 Jun;81(11):5788-806. doi: 10.1128/JVI.00140-07. Epub 2007 Mar 28. J Virol. 2007. PMID: 17392367 Free PMC article.
References
-
- Andre S, Schatz O, Bogner J R, Zeichhardt H, Stoffler-Meilicke M, Jahn H U, Ullrich R, Sonntag A K, Kehm R, Haas J. Detection of antibodies against viral capsid proteins of human herpesvirus 8 in AIDS-associated Kaposi's sarcoma. J Mol Med. 1997;75:145–152. - PubMed
-
- Arvanitakis L, Mesri E A, Nador R G, Said J W, Asch A S, Knowles D M, Cesarman E. Establishment and characterization of a primary effusion (body cavity-based) lymphoma cell line (BC-3) harboring Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8) in the absence of Epstein-Barr virus. Blood. 1996;88:2648–2654. - PubMed
-
- Ballestas M E, Chatis P A, Kaye K M. Efficient persistence of extrachromosomal KSHV DNA mediated by latency-associated nuclear antigen. Science. 1999;284:641–644. - PubMed
-
- Cesarman E, Chang Y, Moore P S, Said J W, Knowles D M. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N Engl J Med. 1995;332:1186–1191. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

