Recognition by human monoclonal antibodies of free and complexed peptides representing the prefusogenic and fusogenic forms of human immunodeficiency virus type 1 gp41
- PMID: 10846104
- PMCID: PMC112119
- DOI: 10.1128/jvi.74.13.6186-6192.2000
Recognition by human monoclonal antibodies of free and complexed peptides representing the prefusogenic and fusogenic forms of human immunodeficiency virus type 1 gp41
Abstract
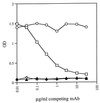
Human immunodeficiency virus type 1 (HIV-1) entry into target cells appears to be triggered when two heptad repeat regions in the ectodomain of gp41 associate, converting the prefusogenic form of gp41 to a fusogenic form. Peptides from these two heptad repeat regions, designated N51 and C43, form a coiled coil consisting of an alpha-helical trimer of heterodimers which approximates the core of the fusogenic form of gp41. To understand the antigenic structures of gp41 in these two configurations, and to examine the specificity of anti-gp41 antibodies produced by HIV-1-infected individuals, human anti-gp41 monoclonal antibodies (MAbs) were tested for their reactivity against N51, C43, and the complex formed by these peptides. Of 11 MAbs, 7 reacted with the complex but with neither of the parent peptides. These MAbs reacted optimally with the N51-C43 complex prepared at a 1:1 ratio and appeared to recognize the fusogenic form of gp41 in which the two heptad repeat regions are associated to form the coiled coil. The existence of antibodies from HIV-infected humans that exclusively recognize the N51-C43 complex constitutes the first proof that the coiled-coil conformation of gp41 exists in vivo and is immunogenic. Two of the 11 MAbs were specific for the hydrophilic loop region of gp41 and failed to react with either peptide alone or with the peptide complex, while the remaining 2 MAbs reacted with peptide C43. One of these two latter MAbs, 98-6, also reacted well with the equimolar N51-C43 complex, while reactivity with C43 by the other MAb, 2F5, was inhibited by even small amounts of N51, suggesting that the interaction of these peptides occludes or disrupts the epitope recognized by MAb 2F5. MAbs 98-6 and 2F5 are also unusual among the MAbs tested in their ability to neutralize multiple primary HIV isolates, although 2F5 displays more broad and potent activity. The data suggest that anti-gp41 neutralizing activity is associated with specificity for a region in C43 which participates in complex formation with N51.
Figures

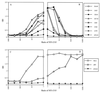
 ) nor C43 alone (▴) bound 126-6*. When unlabeled 126-6 was added to the incubation mixture of N51, C43, and 126-6*, the unlabeled MAb inhibited the binding of 126-6*, showing the specificity of the interaction (□). An irrelevant unlabeled MAb, 1418, to parvovirus B19, did not interfere with the interaction of N51, C43, and 126-6* (○).
) nor C43 alone (▴) bound 126-6*. When unlabeled 126-6 was added to the incubation mixture of N51, C43, and 126-6*, the unlabeled MAb inhibited the binding of 126-6*, showing the specificity of the interaction (□). An irrelevant unlabeled MAb, 1418, to parvovirus B19, did not interfere with the interaction of N51, C43, and 126-6* (○).Similar articles
-
Antigenic properties of the human immunodeficiency virus transmembrane glycoprotein during cell-cell fusion.J Virol. 2002 Dec;76(23):12123-34. doi: 10.1128/jvi.76.23.12123-12134.2002. J Virol. 2002. PMID: 12414953 Free PMC article.
-
Preparation and characterization of four novel monoclonal antibodies specific to N51(L6)C46 polypeptide simulating fusogenic core structure of GP41 subunit of HIV-1.Hybridoma (Larchmt). 2006 Oct;25(5):278-82. doi: 10.1089/hyb.2006.25.278. Hybridoma (Larchmt). 2006. PMID: 17044783
-
Sequestering of the prehairpin intermediate of gp41 by peptide N36Mut(e,g) potentiates the human immunodeficiency virus type 1 neutralizing activity of monoclonal antibodies directed against the N-terminal helical repeat of gp41.J Virol. 2008 Oct;82(20):10032-41. doi: 10.1128/JVI.01050-08. Epub 2008 Jul 30. J Virol. 2008. PMID: 18667502 Free PMC article.
-
Progress towards the development of a HIV-1 gp41-directed vaccine.Curr HIV Res. 2004 Apr;2(2):193-204. doi: 10.2174/1570162043484933. Curr HIV Res. 2004. PMID: 15078183 Review.
-
High throughput screening and characterization of HIV-1 entry inhibitors targeting gp41: theories and techniques.Curr Pharm Des. 2004;10(15):1827-43. doi: 10.2174/1381612043384466. Curr Pharm Des. 2004. PMID: 15180543 Review.
Cited by
-
Genetic evidence that interhelical packing interactions in the gp41 core are critical for transition of the human immunodeficiency virus type 1 envelope glycoprotein to the fusion-active state.J Virol. 2002 Jul;76(14):7356-62. doi: 10.1128/jvi.76.14.7356-7362.2002. J Virol. 2002. PMID: 12072535 Free PMC article.
-
Binding of the 2F5 monoclonal antibody to native and fusion-intermediate forms of human immunodeficiency virus type 1 gp41: implications for fusion-inducing conformational changes.J Virol. 2004 Mar;78(5):2627-31. doi: 10.1128/jvi.78.5.2627-2631.2004. J Virol. 2004. PMID: 14963170 Free PMC article.
-
Neutralization patterns and evolution of sequential HIV type 1 envelope sequences in HIV type 1 subtype B-infected drug-naive individuals.AIDS Res Hum Retroviruses. 2008 Dec;24(12):1507-19. doi: 10.1089/aid.2008.0154. AIDS Res Hum Retroviruses. 2008. PMID: 19018670 Free PMC article.
-
Targeted killing of virally infected cells by radiolabeled antibodies to viral proteins.PLoS Med. 2006 Nov;3(11):e427. doi: 10.1371/journal.pmed.0030427. PLoS Med. 2006. PMID: 17090209 Free PMC article.
-
Distinct conformational states of HIV-1 gp41 are recognized by neutralizing and non-neutralizing antibodies.Nat Struct Mol Biol. 2010 Dec;17(12):1486-91. doi: 10.1038/nsmb.1950. Epub 2010 Nov 14. Nat Struct Mol Biol. 2010. PMID: 21076402 Free PMC article.
References
-
- Barin F, McLane M F, Allan J S, Lee T H, Groopman J E, Essex M. Virus envelope protein of HTLV-III represents major target antigen for antibodies in AIDS patients. Science. 1985;228:1094–1098. - PubMed
-
- Binley J M, Ditzel H J, Barbas III C F, Sullivan N, Sodroski J, Parren P W H I, Burton D R. Human antibody responses to HIV type 1 glycoprotein 41 cloned in phage display libraries suggest three major epitopes are recognized and give evidence for conserved antibody motifs in antigen binding. AIDS Res Hum Retroviruses. 1996;12:911–924. - PubMed
-
- Blacklow S C, Lu M, Kim P S. A trimeric subdomain of the simian immunodeficiency virus envelope glycoprotein. Biochemistry. 1995;34:14955–14962. - PubMed
-
- Cantin R, Fortin J-F, Lamontagne G, Tremblay M. The acquisition of host-derived major histocompatibility complex class II glycoproteins by human immunodeficiency virus type 1 accelerates the process of virus entry and infection in human T lymphoid cells. Blood. 1997;90:1091–1100. - PubMed
-
- Chambers P, Prignle C R, Easton A J. Heptad repeat sequences are located adjacent to hydrophobic regions in several types of virus fusion glycoproteins. J Gen Virol. 1990;71:3075–3080. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

