Anaerobic chlorophyll isocyclic ring formation in Rhodobacter capsulatus requires a cobalamin cofactor
- PMID: 10841582
- PMCID: PMC18774
- DOI: 10.1073/pnas.97.12.6908
Anaerobic chlorophyll isocyclic ring formation in Rhodobacter capsulatus requires a cobalamin cofactor
Abstract
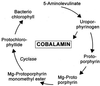
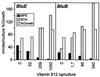
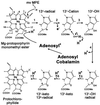
The isocyclic ring of bacteriochlorophyll (BChl) is formed by the conversion of Mg-protoporphyrin monomethyl ester (MPE) to protochlorophyllide (PChlide). Similarities revealed by blast searches with the putative anaerobic MPE-cyclase BchE suggested to us that this protein also uses a cobalamin cofactor. We found that vitamin B(12) (B(12))-requiring mutants of the bluE and bluB genes of Rhodobacter capsulatus, grown without B(12), accumulated Mg-porphyrins. Laser desorption/ionization time-of-flight (LDI-TOF) MS and NMR spectroscopy identified them as MPE and its 3-vinyl-8-ethyl (mvMPE) derivative. An in vivo assay was devised for the cyclase converting MPE to PChlide. Cyclase activity in the B(12)-dependent mutants required B(12) but not protein synthesis. The following reaction mechanism is proposed for this MPE-cyclase reaction. Adenosylcobalamin forms the adenosyl radical, which leads to withdrawal of a hydrogen atom and formation of the benzylic-type 13(1)-radical of MPE. Withdrawal of an electron gives the 13(1)-cation of MPE. Hydroxyl ion attack on the cation gives 13(1)-hydroxy-MPE. Withdrawal of three hydrogen atoms leads successively to 13(1)-keto-MPE, its 13(2)-radical, and cyclization to PChlide.
Figures



Similar articles
-
Mg-protoporphyrin IX monomethyl ester cyclase from Rhodobacter capsulatus: radical SAM-dependent synthesis of the isocyclic ring of bacteriochlorophylls.Biochem J. 2020 Dec 11;477(23):4635-4654. doi: 10.1042/BCJ20200761. Biochem J. 2020. PMID: 33211085
-
A new method for isolating physiologically active Mg-protoporphyrin monomethyl ester, the substrate of the cyclase enzyme of the chlorophyll biosynthetic pathway.Plant Physiol Biochem. 2007 Dec;45(12):932-6. doi: 10.1016/j.plaphy.2007.09.001. Epub 2007 Sep 14. Plant Physiol Biochem. 2007. PMID: 17949988
-
Identification of two homologous genes, chlAI and chlAII, that are differentially involved in isocyclic ring formation of chlorophyll a in the cyanobacterium Synechocystis sp. PCC 6803.J Biol Chem. 2008 Feb 1;283(5):2684-92. doi: 10.1074/jbc.M708954200. Epub 2007 Nov 26. J Biol Chem. 2008. PMID: 18039649
-
bchFNBH bacteriochlorophyll synthesis genes of Rhodobacter capsulatus and identification of the third subunit of light-independent protochlorophyllide reductase in bacteria and plants.J Bacteriol. 1993 Apr;175(8):2414-22. doi: 10.1128/jb.175.8.2414-2422.1993. J Bacteriol. 1993. PMID: 8385667 Free PMC article.
-
Reduction of Chemically Stable Multibonds: Nitrogenase-Like Biosynthesis of Tetrapyrroles.Adv Exp Med Biol. 2017;925:147-161. doi: 10.1007/5584_2016_175. Adv Exp Med Biol. 2017. PMID: 27957709 Review.
Cited by
-
The complete genome sequence of Chlorobium tepidum TLS, a photosynthetic, anaerobic, green-sulfur bacterium.Proc Natl Acad Sci U S A. 2002 Jul 9;99(14):9509-14. doi: 10.1073/pnas.132181499. Epub 2002 Jul 1. Proc Natl Acad Sci U S A. 2002. PMID: 12093901 Free PMC article.
-
Radical S-adenosylmethionine enzymes.Chem Rev. 2014 Apr 23;114(8):4229-317. doi: 10.1021/cr4004709. Epub 2014 Jan 29. Chem Rev. 2014. PMID: 24476342 Free PMC article. Review. No abstract available.
-
(18)O and mass spectrometry in chlorophyll research: Derivation and loss of oxygen atoms at the periphery of the chlorophyll macrocycle during biosynthesis, degradation and adaptation.Photosynth Res. 2000;66(3):159-75. doi: 10.1023/A:1010631505071. Photosynth Res. 2000. PMID: 16228417
-
PpsR, a regulator of heme and bacteriochlorophyll biosynthesis, is a heme-sensing protein.J Biol Chem. 2012 Apr 20;287(17):13850-8. doi: 10.1074/jbc.M112.346494. Epub 2012 Feb 29. J Biol Chem. 2012. PMID: 22378778 Free PMC article.
-
Absence of the cbb3 Terminal Oxidase Reveals an Active Oxygen-Dependent Cyclase Involved in Bacteriochlorophyll Biosynthesis in Rhodobacter sphaeroides.J Bacteriol. 2016 Jul 13;198(15):2056-63. doi: 10.1128/JB.00121-16. Print 2016 Aug 1. J Bacteriol. 2016. PMID: 27215788 Free PMC article.
References
-
- Porra R J, Schafer W, Gad'on N, Katheder I, Drews G, Scheer H. Eur J Biochem. 1996;239:85–92. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

