Receptor-mediated uptake of antigen/heat shock protein complexes results in major histocompatibility complex class I antigen presentation via two distinct processing pathways
- PMID: 10839810
- PMCID: PMC2213527
- DOI: 10.1084/jem.191.11.1957
Receptor-mediated uptake of antigen/heat shock protein complexes results in major histocompatibility complex class I antigen presentation via two distinct processing pathways
Abstract
Heat shock proteins (HSPs) derived from tumors or virally infected cells can stimulate antigen-specific CD8(+) T cell responses in vitro and in vivo. Although this antigenicity is known to arise from HSP-associated peptides presented to the immune system by major histocompatibility complex (MHC) class I molecules, the cell biology underlying this presentation process remains poorly understood. Here we show that HSP 70 binds to the surface of antigen presenting cells by a mechanism with the characteristics of a saturable receptor system. After this membrane interaction, processing and MHC class I presentation of the HSP-associated antigen can occur via either a cytosolic (transporter associated with antigen processing [TAP] and proteasome-dependent) or an endosomal (TAP and proteasome-independent) route, with the preferred pathway determined by the sequence context of the optimal antigenic peptide within the HSP-associated material. These findings not only characterize two highly efficient, specific pathways leading to the conversion of HSP-associated antigens into ligands for CD8(+) T cells, they also imply the existence of a mechanism for receptor-facilitated transmembrane transport of HSP or HSP-associated ligands from the plasma membrane or lumen of endosomes into the cytosol.
Figures
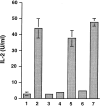
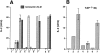




Similar articles
-
Bacterial heat shock proteins promote CD91-dependent class I MHC cross-presentation of chaperoned peptide to CD8+ T cells by cytosolic mechanisms in dendritic cells versus vacuolar mechanisms in macrophages.J Immunol. 2004 May 1;172(9):5277-86. doi: 10.4049/jimmunol.172.9.5277. J Immunol. 2004. PMID: 15100266
-
Direct delivery of the Bordetella pertussis adenylate cyclase toxin to the MHC class I antigen presentation pathway.J Immunol. 1999 Feb 15;162(4):1910-6. J Immunol. 1999. PMID: 9973458
-
Control of cross-presentation during dendritic cell maturation.Eur J Immunol. 2004 Feb;34(2):398-407. doi: 10.1002/eji.200324508. Eur J Immunol. 2004. PMID: 14768044
-
Heat shock proteins in antigen trafficking--implications on antigen presentation to T cells.Int J Hyperthermia. 2009 Dec;25(8):617-25. doi: 10.3109/02656730902902183. Int J Hyperthermia. 2009. PMID: 19551545 Review.
-
Compartmentalization of class II antigen presentation: contribution of cytoplasmic and endosomal processing.Immunol Rev. 2005 Oct;207:206-17. doi: 10.1111/j.0105-2896.2005.00297.x. Immunol Rev. 2005. PMID: 16181338 Review.
Cited by
-
Trial watch: Chemotherapy with immunogenic cell death inducers.Oncoimmunology. 2012 Mar 1;1(2):179-188. doi: 10.4161/onci.1.2.19026. Oncoimmunology. 2012. PMID: 22720239 Free PMC article.
-
High efficiency CD91- and LOX-1-mediated re-presentation of gp96-chaperoned peptides by MHC II molecules.Cancer Immun. 2010 Aug 2;10:7. Cancer Immun. 2010. PMID: 20672796 Free PMC article.
-
Tumor-derived heat shock protein 70-pulsed dendritic cells elicit tumor-specific cytotoxic T lymphocytes (CTLs) and tumor immunity.Cancer Sci. 2004 Mar;95(3):248-53. doi: 10.1111/j.1349-7006.2004.tb02211.x. Cancer Sci. 2004. PMID: 15016325 Free PMC article.
-
GRP94 (gp96) and GRP94 N-terminal geldanamycin binding domain elicit tissue nonrestricted tumor suppression.J Exp Med. 2002 Dec 2;196(11):1447-59. doi: 10.1084/jem.20020436. J Exp Med. 2002. PMID: 12461080 Free PMC article.
-
Experimental study on therapeutic effect of in vivo expression of Cell I-Hep II recombinant polypeptide of fibronectin on murine H22 hepatocellular carcinoma.World J Gastroenterol. 2003 Sep;9(9):1940-5. doi: 10.3748/wjg.v9.i9.1940. World J Gastroenterol. 2003. PMID: 12970880 Free PMC article.
References
-
- Germain R.N. MHC-dependent antigen processing and peptide presentationproviding ligands for T lymphocyte activation. Cell. 1994;76:287–299. - PubMed
-
- Pamer E., Cresswell P. Mechanism of MHC class I-restricted antigen processing. Annu. Rev. Immunol. 1998;16:323–358. - PubMed
-
- Castellino F., Germain R.N. Extensive trafficking of MHC class II-invariant chain complexes in the endocytic pathway and appearance of peptide-loaded class II in multiple compartments. Immunity. 1995;2:73–88. - PubMed
-
- Watts C. Capture and processing of exogenous antigens for presentation on MHC molecules. Annu. Rev. Immunol. 1997;15:821–850. - PubMed
-
- Castellino F., Zappacosta F., Coligan J.E., Germain R.N. Large protein fragments as substrates for endocytic antigen capture by MHC class II molecules. J. Immunol. 1998;161:4048–4057. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

