Protein kinase B regulates T lymphocyte survival, nuclear factor kappaB activation, and Bcl-X(L) levels in vivo
- PMID: 10811865
- PMCID: PMC2193154
- DOI: 10.1084/jem.191.10.1721
Protein kinase B regulates T lymphocyte survival, nuclear factor kappaB activation, and Bcl-X(L) levels in vivo
Abstract
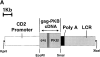
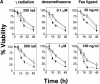
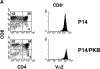
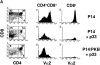
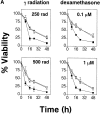
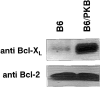
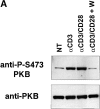
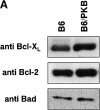
The serine/threonine kinase protein kinase B (PKB)/Akt mediates cell survival in a variety of systems. We have generated transgenic mice expressing a constitutively active form of PKB (gag-PKB) to examine the effects of PKB activity on T lymphocyte survival. Thymocytes and mature T cells overexpressing gag-PKB displayed increased active PKB, enhanced viability in culture, and resistance to a variety of apoptotic stimuli. PKB activity prolonged the survival of CD4(+)CD8(+) double positive (DP) thymocytes in fetal thymic organ culture, but was unable to prevent antigen-induced clonal deletion of thymocytes expressing the major histocompatibility complex class I-restricted P14 T cell receptor (TCR). In mature T lymphocytes, PKB can be activated in response to TCR stimulation, and peptide-antigen-specific proliferation is enhanced in T cells expressing the gag-PKB transgene. Both thymocytes and T cells overexpressing gag-PKB displayed elevated levels of the antiapoptotic molecule Bcl-X(L). In addition, the activation of peripheral T cells led to enhanced nuclear factor (NF)-kappaB activation via accelerated degradation of the NF-kappaB inhibitory protein IkappaBalpha. Our data highlight a physiological role for PKB in promoting survival of DP thymocytes and mature T cells, and provide evidence for the direct association of three major survival molecules (PKB, Bcl-X(L), and NF-kappaB) in vivo in T lymphocytes.
Figures










Similar articles
-
Active protein kinase B regulates TCR responsiveness by modulating cytoplasmic-nuclear localization of NFAT and NF-kappa B proteins.J Immunol. 2004 Apr 15;172(8):4812-20. doi: 10.4049/jimmunol.172.8.4812. J Immunol. 2004. PMID: 15067058
-
Inhibition of nuclear factor kappaB and phosphatidylinositol 3-kinase/Akt is essential for massive hepatocyte apoptosis induced by tumor necrosis factor alpha in mice.Liver Int. 2003 Oct;23(5):386-96. doi: 10.1034/j.1478-3231.2003.00867.x. Liver Int. 2003. PMID: 14708901
-
Constitutively active protein kinase B enhances Lck and Erk activities and influences thymocyte selection and activation.J Immunol. 2003 Aug 1;171(3):1285-96. doi: 10.4049/jimmunol.171.3.1285. J Immunol. 2003. PMID: 12874217
-
Protein kinase C and AKT/protein kinase B in CD4+ T-lymphocytes: new partners in TCR/CD28 signal integration.Mol Immunol. 2002 Jun;38(15):1087-99. doi: 10.1016/s0161-5890(02)00011-1. Mol Immunol. 2002. PMID: 12044776 Review.
-
Intracellular signals of T cell costimulation.Cell Mol Immunol. 2008 Aug;5(4):239-47. doi: 10.1038/cmi.2008.30. Cell Mol Immunol. 2008. PMID: 18761811 Free PMC article. Review.
Cited by
-
Involvement of distinct PKC gene products in T cell functions.Front Immunol. 2012 Aug 6;3:220. doi: 10.3389/fimmu.2012.00220. eCollection 2012. Front Immunol. 2012. PMID: 22888329 Free PMC article.
-
CD28 promotes CD4+ T cell clonal expansion during infection independently of its YMNM and PYAP motifs.J Immunol. 2012 Sep 15;189(6):2909-17. doi: 10.4049/jimmunol.1103231. Epub 2012 Aug 15. J Immunol. 2012. PMID: 22896637 Free PMC article.
-
Constitutively activated Akt-1 is vital for the survival of human monocyte-differentiated macrophages. Role of Mcl-1, independent of nuclear factor (NF)-kappaB, Bad, or caspase activation.J Exp Med. 2001 Jul 16;194(2):113-26. doi: 10.1084/jem.194.2.113. J Exp Med. 2001. PMID: 11457886 Free PMC article.
-
Chimeric antigen receptors combining 4-1BB and CD28 signaling domains augment PI3kinase/AKT/Bcl-XL activation and CD8+ T cell-mediated tumor eradication.Mol Ther. 2010 Feb;18(2):413-20. doi: 10.1038/mt.2009.210. Epub 2009 Sep 22. Mol Ther. 2010. PMID: 19773745 Free PMC article.
-
The AKT-mTOR axis regulates de novo differentiation of CD4+Foxp3+ cells.J Exp Med. 2008 Mar 17;205(3):565-74. doi: 10.1084/jem.20071477. Epub 2008 Feb 18. J Exp Med. 2008. PMID: 18283119 Free PMC article.
References
-
- Sebzda E., Mariathasan S., Ohteki T., Jones R., Bachmann M.F., Ohashi P.S. Selection of the T cell repertoire. Annu. Rev. Immunol. 1999;17:829–874. - PubMed
-
- Takeda S., Rodewald H.-R., Arakawa H., Bluethmann H., Shimizu T. MHC class II molecules are not required for survival of newly generated CD4+ T cells, but affect their long-term life span. Immunity. 1996;5:217–228. - PubMed
-
- Tanchot C., Lemonnier F.A., Pérarnau B., Freitas A.A., Rocha B. Differential requirements for survival and proliferation of CD8 naive or memory T cells. Science. 1997;276:2057–2062. - PubMed
-
- Lenardo M.J., Chan F.K.M., Hornung F., McFarland H., Siegel R., Wang J., Zheng L. Mature T lymphocyte apoptosis-immune regulation in a dynamic and unpredictable environment. Annu. Rev. Immunol. 1999;17:221–253. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

