A core SMRT corepressor complex containing HDAC3 and TBL1, a WD40-repeat protein linked to deafness
- PMID: 10809664
- PMCID: PMC316569
A core SMRT corepressor complex containing HDAC3 and TBL1, a WD40-repeat protein linked to deafness
Abstract
The corepressor SMRT mediates repression by thyroid hormone receptor (TR) as well as other nuclear hormone receptors and transcription factors. Here we report the isolation of a novel SMRT-containing complex from HeLa cells. This complex contains transducin beta-like protein 1 (TBL1), whose gene is mutated in human sensorineural deafness. It also contains HDAC3, a histone deacetylase not previously thought to interact with SMRT. TBL1 displays structural and functional similarities to Tup1 and Groucho corepressors, sharing their ability to interact with histone H3. In vivo, TBL1 is bridged to HDAC3 through SMRT and can potentiate repression by TR. Intriguingly, loss-of-function TRbeta mutations cause deafness in mice and humans. These results define a new TR corepressor complex with a physical link to histone structure and a potential biological link to deafness.
Figures
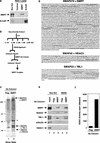
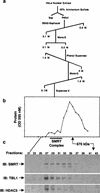
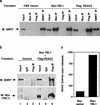
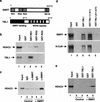
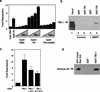
Similar articles
-
Purification and functional characterization of the human N-CoR complex: the roles of HDAC3, TBL1 and TBLR1.EMBO J. 2003 Mar 17;22(6):1336-46. doi: 10.1093/emboj/cdg120. EMBO J. 2003. PMID: 12628926 Free PMC article.
-
Both corepressor proteins SMRT and N-CoR exist in large protein complexes containing HDAC3.EMBO J. 2000 Aug 15;19(16):4342-50. doi: 10.1093/emboj/19.16.4342. EMBO J. 2000. PMID: 10944117 Free PMC article.
-
Analysis of Groucho-histone interactions suggests mechanistic similarities between Groucho- and Tup1-mediated repression.Nucleic Acids Res. 2000 Nov 1;28(21):4189-96. doi: 10.1093/nar/28.21.4189. Nucleic Acids Res. 2000. PMID: 11058116 Free PMC article.
-
N-CoR-HDAC corepressor complexes: roles in transcriptional regulation by nuclear hormone receptors.Curr Top Microbiol Immunol. 2003;274:237-68. doi: 10.1007/978-3-642-55747-7_9. Curr Top Microbiol Immunol. 2003. PMID: 12596910 Review.
-
Nuclear receptor corepressor complexes in cancer: mechanism, function and regulation.Am J Clin Exp Urol. 2014 Oct 2;2(3):169-87. eCollection 2014. Am J Clin Exp Urol. 2014. PMID: 25374920 Free PMC article. Review.
Cited by
-
Chromatin Regulator SPEN/SHARP in X Inactivation and Disease.Cancers (Basel). 2021 Apr 1;13(7):1665. doi: 10.3390/cancers13071665. Cancers (Basel). 2021. PMID: 33916248 Free PMC article. Review.
-
Epigenetic regulation of thyroid hormone-induced adult intestinal stem cell development during anuran metamorphosis.Cell Biosci. 2014 Nov 28;4:73. doi: 10.1186/2045-3701-4-73. eCollection 2014. Cell Biosci. 2014. PMID: 25937894 Free PMC article. Review.
-
The Sox transcriptional factors: Functions during intestinal development in vertebrates.Semin Cell Dev Biol. 2017 Mar;63:58-67. doi: 10.1016/j.semcdb.2016.08.022. Epub 2016 Aug 25. Semin Cell Dev Biol. 2017. PMID: 27567710 Free PMC article. Review.
-
The in vivo role of nuclear receptor corepressors in thyroid hormone action.Biochim Biophys Acta. 2013 Jul;1830(7):3876-81. doi: 10.1016/j.bbagen.2012.07.001. Epub 2012 Jul 16. Biochim Biophys Acta. 2013. PMID: 22801336 Free PMC article. Review.
-
HDAC3 Knockdown Dysregulates Juvenile Hormone and Apoptosis-Related Genes in Helicoverpa armigera.Int J Mol Sci. 2022 Nov 26;23(23):14820. doi: 10.3390/ijms232314820. Int J Mol Sci. 2022. PMID: 36499148 Free PMC article.
References
-
- Alland L, Muhle R, Hou H, Potes J, Chin L, Schreiber-Agus N, DePinho RA. Role for N-CoR and histone deacetylase in Sin3-mediated transcriptional repression. Nature. 1997;387:49–55. - PubMed
-
- Carlson M. Genetics of transcriptional regulation in yeast: Connections to the RNA polymerase II CTD. Annu Rev Cell Dev Biol. 1997;13:1–23. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
