Do glia have heart? Expression and functional role for ether-a-go-go currents in hippocampal astrocytes
- PMID: 10804231
- PMCID: PMC4093789
- DOI: 10.1523/JNEUROSCI.20-10-03915.2000
Do glia have heart? Expression and functional role for ether-a-go-go currents in hippocampal astrocytes
Abstract
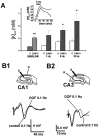
Potassium homeostasis plays an important role in the control of neuronal excitability, and diminished buffering of extracellular K results in neuronal Hyperexcitability and abnormal synchronization. Astrocytes are the cellular elements primarily involved in this process. Potassium uptake into astrocytes occurs, at least in part, through voltage-dependent channels, but the exact mechanisms involved are not fully understood. Although most glial recordings reveal expression of inward rectifier currents (K(IR)), it is not clear how spatial buffering consisting of accumulation and release of potassium may be mediated by exclusively inward potassium fluxes. We hypothesized that a combination of inward and outward rectifiers cooperate in the process of spatial buffering. Given the pharmacological properties of potassium homeostasis (sensitivity to Cs(+)), members of the ether-a-go-go (ERG) channel family widely expressed in the nervous system could underlie part of the process. We used electrophysiological recordings and pharmacological manipulations to demonstrate the expression of ERG-type currents in cultured and in situ hippocampal astrocytes. Specific ERG blockers (dofetilide and E 4031) inhibited hyperpolarization- and depolarization-activated glial currents, and ERG blockade impaired clearance of extracellular potassium with little direct effect on hippocampal neuron excitability. Immunocytochemical analysis revealed ERG protein mostly confined to astrocytes; ERG immunoreactivity was absent in presynaptic and postsynaptic elements, but pronounced in glia surrounding the synaptic cleft. Oligodendroglia did not reveal ERG immunoreactivity. Intense immunoreactivity was also found in perivascular astrocytic end feet at the blood-brain barrier. cDNA amplification showed that cortical astrocytes selectively express HERG1, but not HERG2-3 genes. This study provides insight into a possible physiological role of hippocampal ERG channels and links activation of ERG to control of potassium homeostasis.
Figures


Similar articles
-
Molecular and functional characterization of ERG, KCNQ, and KCNE subtypes in rat stomach smooth muscle.Am J Physiol Gastrointest Liver Physiol. 2002 Feb;282(2):G277-87. doi: 10.1152/ajpgi.00200.2001. Am J Physiol Gastrointest Liver Physiol. 2002. PMID: 11804849
-
High affinity open channel block by dofetilide of HERG expressed in a human cell line.Mol Pharmacol. 1996 Jun;49(6):949-55. Mol Pharmacol. 1996. PMID: 8649354
-
The functional properties of the human ether-à-go-go-like (HELK2) K+ channel.Eur J Neurosci. 2002 Aug;16(3):415-28. doi: 10.1046/j.1460-9568.2002.02079.x. Eur J Neurosci. 2002. PMID: 12193184
-
Ether-à-go-go K+ channels: effective modulators of neuronal excitability.J Physiol. 2018 Mar 1;596(5):769-783. doi: 10.1113/JP275477. Epub 2018 Feb 6. J Physiol. 2018. PMID: 29333676 Free PMC article. Review.
-
Familial and acquired long qt syndrome and the cardiac rapid delayed rectifier potassium current.Clin Exp Pharmacol Physiol. 2000 Oct;27(10):753-66. doi: 10.1046/j.1440-1681.2000.03337.x. Clin Exp Pharmacol Physiol. 2000. PMID: 11022966 Review.
Cited by
-
Probing the outer mouth structure of the HERG channel with peptide toxin footprinting and molecular modeling.Biophys J. 2007 May 15;92(10):3524-40. doi: 10.1529/biophysj.106.097360. Epub 2007 Feb 9. Biophys J. 2007. PMID: 17293393 Free PMC article.
-
In Vitro Blood-Brain Barrier Models for Neuroinfectious Diseases: A Narrative Review.Curr Neuropharmacol. 2024;22(8):1344-1373. doi: 10.2174/1570159X22666231207114346. Curr Neuropharmacol. 2024. PMID: 38073104 Free PMC article. Review.
-
In vitro blood-brain barrier models: current and perspective technologies.J Pharm Sci. 2012 Apr;101(4):1337-54. doi: 10.1002/jps.23022. Epub 2011 Dec 27. J Pharm Sci. 2012. PMID: 22213383 Free PMC article. Review.
-
Genomic biomarkers of SUDEP in brain and heart.Epilepsy Behav. 2014 Sep;38:172-9. doi: 10.1016/j.yebeh.2013.09.019. Epub 2013 Oct 17. Epilepsy Behav. 2014. PMID: 24139807 Free PMC article. Review.
-
Physiology of Astroglia.Physiol Rev. 2018 Jan 1;98(1):239-389. doi: 10.1152/physrev.00042.2016. Physiol Rev. 2018. PMID: 29351512 Free PMC article. Review.
References
-
- Ammann D. Ion sensitive electrodes. Springer; Berlin: 1986.
-
- Araque A, Parpura V, Sanzgiri RP, Haydon PG. Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci. 1999;22:208–215. - PubMed
-
- Ballanyi K, Branchereau P, Champagnat J, Fortin G, Velluti J. Extracellular potassium, glial and neuronal potentials in the solitary complex of rat brainstem slices. Brain Res. 1993;607:99–107. - PubMed
-
- Bennett SA, Stevenson B, Staines WA, Roberts DC. Periodic acid-Schiff (PAS)-positive deposits in brain following kainic acid-induced seizures: relationships to fos induction, neuronal necrosis, reactive gliosis, and blood–brain barrier breakdown. Acta Neuropathol Berl. 1995;89:126–138. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

