Vascular endothelial growth factor receptor-3 in lymphangiogenesis in wound healing
- PMID: 10793061
- PMCID: PMC1876915
- DOI: 10.1016/S0002-9440(10)65021-3
Vascular endothelial growth factor receptor-3 in lymphangiogenesis in wound healing
Abstract
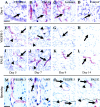
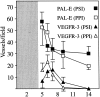
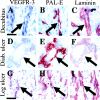
Vascular endothelial growth factor receptor-3 (VEGFR-3) is essential for embryonic cardiovascular development, but thereafter becomes confined to the lymphatic endothelium in adult tissues. We have here studied VEGFR-3 expression in experimental wounds of pigs and chronic inflammatory wounds of humans. In healing incisional and punch biopsy wounds made in the dorsal skin of pigs, angiogenic blood vessels, identified by use of the blood vascular endothelial markers vWF and PAL-E and the basal lamina protein laminin, developed into the granulation tissue stroma from day 4 onward, being most abundant on days 5 and 6 and regressing thereafter. VEGFR-3-positive vessels were observed in the granulation tissue from day 5 onward. These vessels were distinct from the PAL-E/laminin/vWF-positive vessels and fewer in number, and they appeared to sprout from pre-existing VEGFR-3-positive lymphatic vessels at the wound edge. Unlike the blood vessels, very few VEGFR-3-positive lymphatic vessels persisted on day 9 and none on day 14. In chronic wounds such as ulcers and decubitus wounds of the lower extremity of humans, VEGFR-3 was also weakly expressed in the vascular endothelium. Our results suggest that transient lymphangiogenesis occurs in parallel with angiogenesis in healing wounds and that VEGFR-3 becomes up-regulated in blood vessel endothelium in chronic inflammatory wounds.
Figures

Similar articles
-
Expression of the vascular endothelial growth factor C receptor VEGFR-3 in lymphatic endothelium of the skin and in vascular tumors.Am J Pathol. 1998 Aug;153(2):395-403. doi: 10.1016/S0002-9440(10)65583-6. Am J Pathol. 1998. PMID: 9708800 Free PMC article.
-
Expression of VEGFR-3 and 5'-nase in regenerating lymphatic vessels of the cutaneous wound healing.Microsc Res Tech. 2004 Jun 15;64(3):279-86. doi: 10.1002/jemt.20082. Microsc Res Tech. 2004. PMID: 15452895
-
VEGFR-3 in adult angiogenesis.J Pathol. 2001 Nov;195(4):490-7. doi: 10.1002/path.969. J Pathol. 2001. PMID: 11745682
-
Lymphatic versus blood vascular endothelial growth factors and receptors in humans.Microsc Res Tech. 2001 Oct 15;55(2):108-21. doi: 10.1002/jemt.1162. Microsc Res Tech. 2001. PMID: 11596156 Review.
-
Angiogenesis in wound healing and tumor metastasis.Behring Inst Mitt. 1993 Aug;(92):258-72. Behring Inst Mitt. 1993. PMID: 7504453 Review.
Cited by
-
Epigenetic regulation of the lineage specificity of primary human dermal lymphatic and blood vascular endothelial cells.Angiogenesis. 2021 Feb;24(1):67-82. doi: 10.1007/s10456-020-09743-9. Epub 2020 Sep 12. Angiogenesis. 2021. PMID: 32918672 Free PMC article.
-
Generation of pure lymphatic endothelial cells from human pluripotent stem cells and their therapeutic effects on wound repair.Sci Rep. 2015 Jun 12;5:11019. doi: 10.1038/srep11019. Sci Rep. 2015. PMID: 26066093 Free PMC article.
-
Advances in Molecular Regulation of Prostate Cancer Cells by Top Natural Products of Malaysia.Curr Issues Mol Biol. 2023 Feb 9;45(2):1536-1567. doi: 10.3390/cimb45020099. Curr Issues Mol Biol. 2023. PMID: 36826044 Free PMC article. Review.
-
Biomarkers of lymphatic function and disease: state of the art and future directions.Mol Diagn Ther. 2007;11(4):227-38. doi: 10.1007/BF03256244. Mol Diagn Ther. 2007. PMID: 17705577 Review.
-
Insomnia Complaints and Perceived Immune Fitness in Young Adults with and without Self-Reported Impaired Wound Healing.Medicina (Kaunas). 2022 Aug 4;58(8):1049. doi: 10.3390/medicina58081049. Medicina (Kaunas). 2022. PMID: 36013516 Free PMC article.
References
-
- Folkman J: Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med 1995, 1:27-31 - PubMed
-
- Folkman J: Seminars in medicine of the Beth Israel Hospital, Boston: clinical applications of research on angiogenesis. N Engl J Med 1995, 333:1757-1763 - PubMed
-
- Ferrara N: Molecular and biological properties of vascular endothelial growth factor. J Mol Med 1999, 77:527-543 - PubMed
-
- Shibuya M, Ito N, Claesson-Welsh L: Structure and function of vascular endothelial growth factor receptor-1 and -2. Curr Top Microbiol Immunol 1999, 237:59-83 - PubMed
-
- Korpelainen EI, Alitalo K: Signaling angiogenesis and lymphangiogenesis. Curr Opin Cell Biol 1998, 10:159-164 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

