Alymphoplasia (aly)-type nuclear factor kappaB-inducing kinase (NIK) causes defects in secondary lymphoid tissue chemokine receptor signaling and homing of peritoneal cells to the gut-associated lymphatic tissue system
- PMID: 10790423
- PMCID: PMC2213441
- DOI: 10.1084/jem.191.9.1477
Alymphoplasia (aly)-type nuclear factor kappaB-inducing kinase (NIK) causes defects in secondary lymphoid tissue chemokine receptor signaling and homing of peritoneal cells to the gut-associated lymphatic tissue system
Abstract
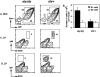
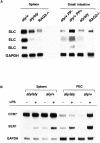
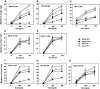
Alymphoplasia (aly) mice, which carry a point mutation in the nuclear factor kappaB-inducing kinase (NIK) gene, are characterized by the systemic absence of lymph nodes and Peyer's patches, disorganized splenic and thymic architectures, and immunodeficiency. Another unique feature of aly/aly mice is that their peritoneal cavity contains more B1 cells than normal and aly/+ mice. Transfer experiments of peritoneal lymphocytes from aly/aly mice into recombination activating gene (RAG)-2(-/-) mice revealed that B and T cells fail to migrate to other lymphoid tissues, particularly to the gut-associated lymphatic tissue system. In vivo homing defects of aly/aly peritoneal cells correlated with reduction of their in vitro chemotactic responses to secondary lymphoid tissue chemokine (SLC) and B lymphocyte chemoattractant (BLC). The migration defect of aly/aly lymphocytes was not due to a lack of expression of chemokines and their receptors, but rather to impaired signal transduction downstream of the receptors for SLC, indicating that NIK is involved in the chemokine signaling pathway known to couple only with G proteins. The results showed that the reduced serum levels of immunoglobulins (Igs) and the absence of class switch to IgA in aly/aly mice are due, at least in part, to a migration defect of lymphocytes to the proper microenvironment where B cells proliferate and differentiate into Ig-producing cells.
Figures

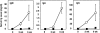


Similar articles
-
The donor splice site mutation in NFkappaB-inducing kinase of alymphoplasia (aly/aly) mice.Immunogenetics. 2003 Jan;54(10):693-8. doi: 10.1007/s00251-002-0517-x. Epub 2002 Dec 3. Immunogenetics. 2003. PMID: 12557055
-
Alymphoplasia is caused by a point mutation in the mouse gene encoding Nf-kappa b-inducing kinase.Nat Genet. 1999 May;22(1):74-7. doi: 10.1038/8780. Nat Genet. 1999. PMID: 10319865
-
Defects of somatic hypermutation and class switching in alymphoplasia (aly) mutant mice.Int Immunol. 1996 Jul;8(7):1067-75. doi: 10.1093/intimm/8.7.1067. Int Immunol. 1996. PMID: 8757952
-
Regulation of secondary lymphoid organ development by the nuclear factor-kappaB signal transduction pathway.Immunol Rev. 2003 Oct;195:91-105. doi: 10.1034/j.1600-065x.2003.00064.x. Immunol Rev. 2003. PMID: 12969313 Review.
-
Generation, expansion, migration and activation of mouse B1 cells.Immunol Rev. 2000 Aug;176:205-15. doi: 10.1034/j.1600-065x.2000.00604.x. Immunol Rev. 2000. PMID: 11043779 Review.
Cited by
-
Regulation of hematopoiesis by chemokine family members.Int J Hematol. 2001 Jul;74(1):9-17. doi: 10.1007/BF02982544. Int J Hematol. 2001. PMID: 11530812 Review.
-
In situ IgM production and clonal expansion of B-1 cells in peritoneal cavity promote elimination of C. albicans infection in IgH transgenic mice with VH derived from a natural antibody.PLoS One. 2013;8(4):e60779. doi: 10.1371/journal.pone.0060779. Epub 2013 Apr 2. PLoS One. 2013. PMID: 23565274 Free PMC article.
-
B cell superantigens: a microbe's answer to innate-like B cells and natural antibodies.Springer Semin Immunopathol. 2005 Mar;26(4):463-84. doi: 10.1007/s00281-004-0190-2. Springer Semin Immunopathol. 2005. PMID: 15633015 Review.
-
Bone marrow-specific knock-in of a non-activatable Ikkα kinase mutant influences haematopoiesis but not atherosclerosis in Apoe-deficient mice.PLoS One. 2014 Feb 3;9(2):e87452. doi: 10.1371/journal.pone.0087452. eCollection 2014. PLoS One. 2014. PMID: 24498325 Free PMC article.
-
Carboxyl terminus of HSC70-interacting protein (CHIP) down-regulates NF-κB-inducing kinase (NIK) and suppresses NIK-induced liver injury.J Biol Chem. 2015 May 1;290(18):11704-14. doi: 10.1074/jbc.M114.635086. Epub 2015 Mar 19. J Biol Chem. 2015. PMID: 25792747 Free PMC article.
References
-
- De Togni P., Goellner J., Ruddle N.H., Streeter P.R., Fick A., Mariathasan S., Smith S.C., Carlson R., Shornick L.P., Strauss-Schoenberger J. Abnormal development of peripheral lymphoid organs in mice deficient in lymphotoxin. Science. 1994;264:703–707. - PubMed
-
- Banks T.A., Rouse B.T., Kerley M.K., Blair P.J., Godfrey V.L., Kuklin N.A., Bouley D.M., Thomas J., Kanangat S., Mucenski M.L. Lymphotoxin-α-deficient mice. Effects on secondary lymphoid organ development and humoral immune responsiveness. J. Immunol. 1995;155:1685–1693. - PubMed
-
- Koni P.A., Sacca R., Lawton P., Browning J.L., Ruddle N.H., Flavell R.A. Distinct roles in lymphoid organogenesis for lymphotoxins α and β revealed in lymphotoxin β-deficient mice. Immunity. 1997;6:491–500. - PubMed
-
- Mauri D.N., Ebner R., Montgomery R.I., Kochel K.D., Cheung T.C., Yu G.L., Ruben S., Murphy M., Eisenberg R.J., Cohen G.H. LIGHT, a new member of the TNF superfamily, and lymphotoxin α are ligands for herpesvirus entry mediator. Immunity. 1998;8:21–30. - PubMed
-
- Pasparakis M., Alexopoulou L., Episkopou V., Kollias G. Immune and inflammatory responses in TNF alpha–deficient micea critical requirement for TNF alpha in the formation of primary B cell follicles, follicular dendritic cell networks and germinal centers, and in the maturation of the humoral immune response. J. Exp. Med. 1996;184:1397–1411. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

