Adhesion to the extracellular matrix regulates the coupling of the small GTPase Rac to its effector PAK
- PMID: 10790367
- PMCID: PMC305684
- DOI: 10.1093/emboj/19.9.2008
Adhesion to the extracellular matrix regulates the coupling of the small GTPase Rac to its effector PAK
Abstract
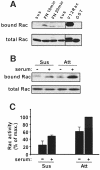
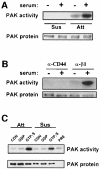
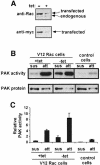
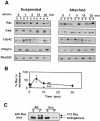
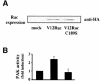
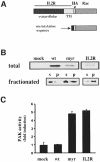
The small GTPase Rac regulates cytoskeletal organization, cell cycle progression, gene expression and oncogenic transformation, processes that depend upon both soluble growth factors and adhesion to the extracellular matrix (ECM). We now show that growth factors and adhesion to the ECM both contribute independently and approximately equally to Rac activation. However, activated Rac in non-adherent cells failed to stimulate the Rac effector PAK. V12 Rac or Rac activated by serum translocated to the membrane fraction of adherent cells but remained mainly cytoplasmic in suspended cells. An activated Rac mutant lacking a membrane-targeting sequence did not activate PAK in adherent cells, while mutations that forced membrane targeting restored PAK activation in suspended cells. In vitro, V12 Rac showed greater binding to membranes from adherent relative to suspended cells, indicating that cell adhesion regulated membrane binding sites for Rac. These results show that ECM regulates the ability of Rac to couple with PAK via an effect on membrane binding sites that facilitate their interaction.
Figures

Similar articles
-
Regulation of the protein kinase Raf-1 by oncogenic Ras through phosphatidylinositol 3-kinase, Cdc42/Rac and Pak.Curr Biol. 2000 Mar 9;10(5):281-4. doi: 10.1016/s0960-9822(00)00359-6. Curr Biol. 2000. PMID: 10712905
-
Tyrosine 221 in Crk regulates adhesion-dependent membrane localization of Crk and Rac and activation of Rac signaling.EMBO J. 2002 Sep 2;21(17):4571-82. doi: 10.1093/emboj/cdf446. EMBO J. 2002. PMID: 12198159 Free PMC article.
-
Rac-PAK signaling stimulates extracellular signal-regulated kinase (ERK) activation by regulating formation of MEK1-ERK complexes.Mol Cell Biol. 2002 Sep;22(17):6023-33. doi: 10.1128/MCB.22.17.6023-6033.2002. Mol Cell Biol. 2002. PMID: 12167697 Free PMC article.
-
Adhesion signaling: PAK meets Rac on solid ground.Curr Biol. 2000 Jul 13;10(14):R535-7. doi: 10.1016/s0960-9822(00)00588-1. Curr Biol. 2000. PMID: 10898998 Review.
-
Involvement of Rac/Cdc42/PAK pathway in cytoskeletal rearrangements.Acta Biochim Pol. 2009;56(2):225-34. Epub 2009 Jun 10. Acta Biochim Pol. 2009. PMID: 19513348 Review.
Cited by
-
Activation of endothelial ras-related C3 botulinum toxin substrate 1 (Rac1) improves post-stroke recovery and angiogenesis via activating Pak1 in mice.Exp Neurol. 2019 Dec;322:113059. doi: 10.1016/j.expneurol.2019.113059. Epub 2019 Sep 6. Exp Neurol. 2019. PMID: 31499064 Free PMC article. Review.
-
MHC class II deprivation impairs CD4 T cell motility and responsiveness to antigen-bearing dendritic cells in vivo.Proc Natl Acad Sci U S A. 2007 Apr 24;104(17):7181-6. doi: 10.1073/pnas.0608299104. Epub 2007 Apr 13. Proc Natl Acad Sci U S A. 2007. PMID: 17435166 Free PMC article.
-
alpha5 integrin signaling regulates the formation of spines and synapses in hippocampal neurons.J Biol Chem. 2007 Mar 9;282(10):6929-35. doi: 10.1074/jbc.M610981200. Epub 2007 Jan 9. J Biol Chem. 2007. PMID: 17213186 Free PMC article.
-
Rac1-induced cell migration requires membrane recruitment of the nuclear oncogene SET.EMBO J. 2007 Jan 24;26(2):336-45. doi: 10.1038/sj.emboj.7601518. EMBO J. 2007. PMID: 17245428 Free PMC article.
-
Protein tyrosine phosphatase α phosphotyrosyl-789 binds BCAR3 to position Cas for activation at integrin-mediated focal adhesions.Mol Cell Biol. 2012 Sep;32(18):3776-89. doi: 10.1128/MCB.00214-12. Epub 2012 Jul 16. Mol Cell Biol. 2012. PMID: 22801373 Free PMC article.
References
-
- Anand-Apte B., Zetter,B.R., Viswanathan,A., Qui,R.G., Chen,J., Ruggieri,R. and Symons,M. (1997) Platelet-derived growth factor and fibronectin stimulated migration are differentially regulated by the Rac and extracellular signal regulated kinase pathways. J. Biol. Chem., 272, 30688–30692. - PubMed
-
- Bokoch G.M., Bohl,B.P. and Chuang,T.H. (1994) Guanine nucleotide exchange regulates membrane translocation of Rac/Rho GTP-binding proteins. J. Biol. Chem., 269, 31674–31679. - PubMed
-
- Chong L.D., Traynor-Kaplan,A., Bokoch,G.M. and Schwartz,M.A. (1994) The small GTP-binding protein Rho regulates a phosphatidylinositol 4-phosphate 5-kinase in mammalian cells. Cell, 79, 507–513. - PubMed
-
- Choy E., Chiu,V.K., Siletti,J., Feoktisov,M., Morimoto,T., Michaelson,D., Ivanov,I.E. and Phillips,M.R. (1999) Endomembrane trafficking of Ras: the CAAX motif targets proteins to the ER and Golgi. Cell, 98, 69–80. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

