Nuclear structure in normal and Bloom syndrome cells
- PMID: 10779560
- PMCID: PMC25808
- DOI: 10.1073/pnas.090525897
Nuclear structure in normal and Bloom syndrome cells
Abstract
Bloom syndrome (BS) is a rare cancer-predisposing disorder in which the cells of affected persons have a high frequency of somatic mutation and genomic instability. BLM, the protein altered in BS, is a RecQ DNA helicase. This report shows that BLM is found in the nucleus of normal human cells in the nuclear domain 10 or promyelocytic leukemia nuclear bodies. These structures are punctate depots of proteins disrupted upon viral infection and in certain human malignancies. BLM is found primarily in nuclear domain 10 except during S phase when it colocalizes with the Werner syndrome gene product, WRN, in the nucleolus. BLM colocalizes with a select subset of telomeres in normal cells and with large telomeric clusters seen in simian virus 40-transformed normal fibroblasts. During S phase, BS cells expel micronuclei containing sites of DNA synthesis. BLM is likely to be part of a DNA surveillance mechanism operating during S phase.
Figures
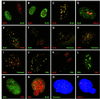
Similar articles
-
Localization of the Bloom syndrome helicase to punctate nuclear structures and the nuclear matrix and regulation during the cell cycle: comparison with the Werner's syndrome helicase.Mol Carcinog. 1999 Dec;26(4):261-73. doi: 10.1002/(sici)1098-2744(199912)26:4<261::aid-mc5>3.0.co;2-a. Mol Carcinog. 1999. PMID: 10569803
-
Telomere and ribosomal DNA repeats are chromosomal targets of the bloom syndrome DNA helicase.BMC Cell Biol. 2003 Oct 27;4:15. doi: 10.1186/1471-2121-4-15. BMC Cell Biol. 2003. PMID: 14577841 Free PMC article.
-
POT1 stimulates RecQ helicases WRN and BLM to unwind telomeric DNA substrates.J Biol Chem. 2005 Sep 16;280(37):32069-80. doi: 10.1074/jbc.M505211200. Epub 2005 Jul 18. J Biol Chem. 2005. PMID: 16030011
-
Werner syndrome: entering the helicase era.Bioessays. 1996 Dec;18(12):1025-7. doi: 10.1002/bies.950181214. Bioessays. 1996. PMID: 8976161 Review.
-
[Bloom syndrome].Nihon Rinsho. 2000 Jul;58(7):1460-6. Nihon Rinsho. 2000. PMID: 10921324 Review. Japanese.
Cited by
-
Impairment of osteoblast differentiation due to proliferation-independent telomere dysfunction in mouse models of accelerated aging.Aging Cell. 2012 Aug;11(4):704-13. doi: 10.1111/j.1474-9726.2012.00838.x. Epub 2012 Jun 11. Aging Cell. 2012. PMID: 22621437 Free PMC article.
-
Regulation and localization of the Bloom syndrome protein in response to DNA damage.J Cell Biol. 2001 Apr 16;153(2):367-80. doi: 10.1083/jcb.153.2.367. J Cell Biol. 2001. PMID: 11309417 Free PMC article.
-
Deficiency of Bloom syndrome helicase activity is radiomimetic.Cancer Biol Ther. 2008 Nov;7(11):1783-6. doi: 10.4161/cbt.7.11.6779. Epub 2008 Nov 4. Cancer Biol Ther. 2008. PMID: 18787401 Free PMC article.
-
BLM helicase suppresses recombination at G-quadruplex motifs in transcribed genes.Nat Commun. 2018 Jan 18;9(1):271. doi: 10.1038/s41467-017-02760-1. Nat Commun. 2018. PMID: 29348659 Free PMC article.
-
The C-terminal domain of the Bloom syndrome DNA helicase is essential for genomic stability.BMC Cell Biol. 2001;2:11. doi: 10.1186/1471-2121-2-11. Epub 2001 Jul 2. BMC Cell Biol. 2001. PMID: 11472631 Free PMC article.
References
-
- German J. Medicine. 1993;72:393–406. - PubMed
-
- German J, Ellis N A. In: The Genetic Basis of Human Cancer. Vogelstein B, Kinzler K W, editors. New York: McGraw–Hill; 1998. pp. 301–315.
-
- Ellis N A, Groden J, Ye T-Z, Straughen J, Lennon D, Ciocci S, Proytcheva M, German J. Cell. 1995;83:655–666. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

