E2F4 and E2F1 have similar proliferative properties but different apoptotic and oncogenic properties in vivo
- PMID: 10779331
- PMCID: PMC85634
- DOI: 10.1128/MCB.20.10.3417-3424.2000
E2F4 and E2F1 have similar proliferative properties but different apoptotic and oncogenic properties in vivo
Abstract
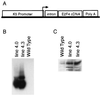
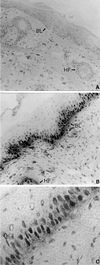
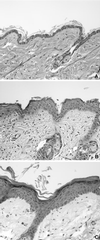
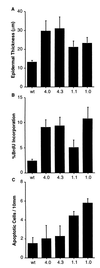
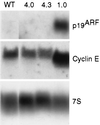
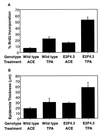
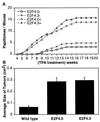
Loss of retinoblastoma (Rb) tumor suppressor function, as occurs in many cancers, leads to uncontrolled proliferation, an increased propensity to undergo apoptosis, and tumorigenesis. Rb negatively regulates multiple E2F transcription factors, but the role of the different E2F family members in manifesting the cellular response to Rb inactivation is unclear. To study the effect of deregulated E2F4 activity on cell growth control and tumorigenesis, transgenic mouse lines expressing the E2F4 gene under the control of a keratin 5 (K5) promoter were developed, and their phenotypes were compared to those of previously generated K5 E2F1 transgenic mice. In contrast to what has been observed in vitro, ectopically expressed E2F4 was found to localize to the nucleus and induce proliferation to an extent similar to that induced by E2F1 in transgenic tissue. Unlike E2F1, E2F4 does not induce apoptosis, and this correlates with the differential abilities of these two E2F species to stimulate p19(ARF) expression in vivo. To examine the role of E2F4 in tumor development, the mouse skin two-stage carcinogenesis model was utilized. Unlike E2F1 transgenic mice, E2F4 transgenic mice developed skin tumors with a decreased latency and increased incidence compared to those characteristics in wild-type controls. These findings demonstrate that while the effects of E2F1 and E2F4 on cell proliferation in vivo are similar, their apoptotic and oncogenic properties are quite different.
Figures

Similar articles
-
Deregulated expression of E2F1 induces hyperplasia and cooperates with ras in skin tumor development.Oncogene. 1998 Mar 12;16(10):1267-76. doi: 10.1038/sj.onc.1201666. Oncogene. 1998. PMID: 9546428
-
E2F4-RB and E2F4-p107 complexes suppress gene expression by transforming growth factor beta through E2F binding sites.Proc Natl Acad Sci U S A. 1997 May 13;94(10):4948-53. doi: 10.1073/pnas.94.10.4948. Proc Natl Acad Sci U S A. 1997. PMID: 9144170 Free PMC article.
-
Deregulated expression of DP1 induces epidermal proliferation and enhances skin carcinogenesis.Mol Carcinog. 2001 Jun;31(2):90-100. doi: 10.1002/mc.1044. Mol Carcinog. 2001. PMID: 11429786
-
The cellular effects of E2F overexpression.Curr Top Microbiol Immunol. 1996;208:79-93. doi: 10.1007/978-3-642-79910-5_4. Curr Top Microbiol Immunol. 1996. PMID: 8575214 Review.
-
Upregulation of E2F transcription factors in chemically induced mouse skin tumors.Int J Oncol. 1999 Aug;15(2):387-90. doi: 10.3892/ijo.15.2.387. Int J Oncol. 1999. PMID: 10402252 Review.
Cited by
-
MDM2 induces hyperplasia and premalignant lesions when expressed in the basal layer of the epidermis.EMBO J. 2000 Oct 2;19(19):5135-47. doi: 10.1093/emboj/19.19.5135. EMBO J. 2000. PMID: 11013216 Free PMC article.
-
The Histone Demethylase PHF8 Is Essential for Endothelial Cell Migration.PLoS One. 2016 Jan 11;11(1):e0146645. doi: 10.1371/journal.pone.0146645. eCollection 2016. PLoS One. 2016. PMID: 26751588 Free PMC article.
-
Novel functions for the transcription factor E2F4 in development and disease.Cell Cycle. 2016 Dec;15(23):3183-3190. doi: 10.1080/15384101.2016.1234551. Epub 2016 Oct 18. Cell Cycle. 2016. PMID: 27753528 Free PMC article. Review.
-
The role of the E2F transcription factor family in UV-induced apoptosis.Int J Mol Sci. 2011;12(12):8947-60. doi: 10.3390/ijms12128947. Epub 2011 Dec 6. Int J Mol Sci. 2011. PMID: 22272113 Free PMC article. Review.
-
Constitutive E2F expression in tobacco plants exhibits altered cell cycle control and morphological change in a cell type-specific manner.Plant Physiol. 2003 Aug;132(4):2012-22. doi: 10.1104/pp.103.025080. Plant Physiol. 2003. PMID: 12913157 Free PMC article.
References
-
- Bates S, Phillips A C, Clark P A, Stott F, Peters G, Ludwig R L, Vousden K H. p14ARF links the tumour suppressors RB and p53. Nature. 1998;395:124–125. . (Letter.) - PubMed
-
- Beijersbergen R L, Kerkhoven R M, Zhu L, Carlee L, Voorhoeve P M, Bernards R. E2F-4, a new member of the E2F gene family, has oncogenic activity and associates with p107 in vivo. Genes Dev. 1994;8:2680–2690. - PubMed
-
- Dover R, Wright N A. The cell proliferation kinetics of the epidermis. In: Goldsworth L A, editor. Physiology, biochemistry, and molecular biology of the skin. Vol. 1. New York, N.Y: Oxford University Press; 1991. pp. 239–265.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
