The role of virus-specific CD8(+) cells in liver damage and viral control during persistent hepatitis B virus infection
- PMID: 10770795
- PMCID: PMC2193131
- DOI: 10.1084/jem.191.8.1269
The role of virus-specific CD8(+) cells in liver damage and viral control during persistent hepatitis B virus infection
Abstract
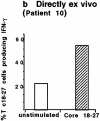
Hepatitis B virus (HBV) is a noncytopathic virus, and the recognition of infected hepatocytes by HBV-specific CD8 cells has been assumed to be the central mechanism causing both liver damage and virus control. To understand the role of cytotoxic T cells in the pathogenesis of HBV infection, we used functional assays that require T cell expansion in vitro and human histocompatibility leukocyte antigen (HLA)-peptide tetramers that allow direct ex vivo quantification of circulating and liver-infiltrating HBV-specific CD8 cells. Two groups of patients with persistent HBV infection were studied: one without liver inflammation and HBV replication, the other with liver inflammation and a high level of HBV replication. Contrary to expectation, a high frequency of intrahepatic HBV-specific CD8 cells was found in the absence of hepatic immunopathology. In contrast, virus-specific T cells were more diluted among liver infiltrates in viremic patients, but their absolute number was similar because of the massive cellular infiltration. Furthermore, inhibition of HBV replication was associated with the presence of a circulating reservoir of CD8(+) cells able to expand after specific virus recognition that was not detectable in highly viremic patients with liver inflammation. These results show that in the presence of an effective HBV-specific CD8 response, inhibition of virus replication can be independent of liver damage. When the HBV-specific CD8 response is unable to control virus replication, it may contribute to liver pathology not only directly but by causing the recruitment of nonvirus-specific T cells.
Figures





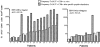
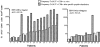


Similar articles
-
Role of hepatitis B virus specific cytotoxic T cells in liver damage and viral control.Antiviral Res. 2003 Oct;60(2):61-6. doi: 10.1016/j.antiviral.2003.08.012. Antiviral Res. 2003. PMID: 14638399
-
[Direct analysis of HBV-specific CD8+ lymphocyte by tetrameric HLA-A2/core 18-27 complex in chronic Hepatitis B].Taehan Kan Hakhoe Chi. 2002 Jun;8(2):139-48. Taehan Kan Hakhoe Chi. 2002. PMID: 12499799 Korean.
-
Dynamic programmed death 1 expression by virus-specific CD8 T cells correlates with the outcome of acute hepatitis B.Gastroenterology. 2008 Jun;134(7):1938-49, 1949.e1-3. doi: 10.1053/j.gastro.2008.03.037. Epub 2008 Mar 22. Gastroenterology. 2008. PMID: 18455515
-
Liver-Resident Memory CD8+ T Cells: Possible Roles in Chronic HBV Infection.Int J Mol Sci. 2020 Dec 30;22(1):283. doi: 10.3390/ijms22010283. Int J Mol Sci. 2020. PMID: 33396596 Free PMC article. Review.
-
HBV-Specific CD8+ T-Cell Tolerance in the Liver.Front Immunol. 2021 Aug 6;12:721975. doi: 10.3389/fimmu.2021.721975. eCollection 2021. Front Immunol. 2021. PMID: 34421926 Free PMC article. Review.
Cited by
-
Regulation of T cell function by microRNA-720.Sci Rep. 2015 Jul 22;5:12159. doi: 10.1038/srep12159. Sci Rep. 2015. PMID: 26199080 Free PMC article.
-
Multifunctional CD4 T cell responses in patients with active tuberculosis.Sci Rep. 2012;2:216. doi: 10.1038/srep00216. Epub 2012 Jan 10. Sci Rep. 2012. PMID: 22355730 Free PMC article.
-
Relapse rate and associated-factor of recurrence after stopping NUCs therapy with different prolonged consolidation therapy in HBeAg positive CHB patients.PLoS One. 2013 Jul 3;8(7):e68568. doi: 10.1371/journal.pone.0068568. Print 2013. PLoS One. 2013. PMID: 23844222 Free PMC article.
-
Current progress in the development of therapeutic vaccines for chronic hepatitis B virus infection.Iran J Basic Med Sci. 2016 Jul;19(7):692-704. Iran J Basic Med Sci. 2016. PMID: 27635192 Free PMC article. Review.
-
Macrophage migration inhibitory factor receptor CD74 expression is associated with expansion and differentiation of effector T cells in COVID-19 patients.Front Immunol. 2023 Oct 25;14:1236374. doi: 10.3389/fimmu.2023.1236374. eCollection 2023. Front Immunol. 2023. PMID: 37946732 Free PMC article.
References
-
- Di Marco V., Lo Iacono O., Camma C., Vaccaro A., Giunta M., Martorana G., Fuschi P., Almasio P.L., Craxi A. The long-term course of chronic hepatitis B. Hepatology. 1999;30:257–264. - PubMed
-
- Chisari F., Ferrari C. Hepatitis B virus immunopathogenesis. Annu. Rev. Immunol. 1995;13:29–60. - PubMed
-
- Moriyama T., Guilhot S., Klopchin K., Moss B., Pinkert C.A., Palmiter R.D., Brinster R.L., Kanagawa O., Chisari F.V. Immunobiology and pathogenesis of hepatocellular injury in hepatitis B virus transgenic mice. Science. 1990;248:361–364. - PubMed
-
- Nayersina R., Fowler P., Guilhot S., Missale G., Cerny A., Schlicht H.J., Vitiello A., Chesnut R., Person J.L., Redeker A.J., Chisari F.V. HLA-A2 restricted cytotoxic T lymphocyte responses to multiple hepatitis B surface antigen epitopes during hepatitis B virus infection. J. Immunol. 1993;150:4659–4671. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

