Multiple forces contribute to cell sheet morphogenesis for dorsal closure in Drosophila
- PMID: 10769037
- PMCID: PMC2175161
- DOI: 10.1083/jcb.149.2.471
Multiple forces contribute to cell sheet morphogenesis for dorsal closure in Drosophila
Abstract
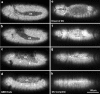
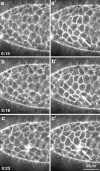
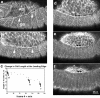
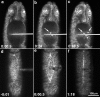
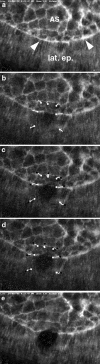
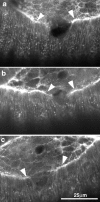
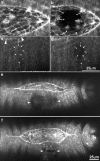
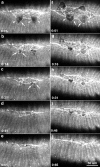
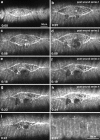
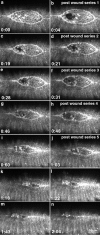
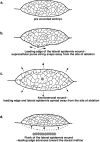
The molecular and cellular bases of cell shape change and movement during morphogenesis and wound healing are of intense interest and are only beginning to be understood. Here, we investigate the forces responsible for morphogenesis during dorsal closure with three approaches. First, we use real-time and time-lapsed laser confocal microscopy to follow actin dynamics and document cell shape changes and tissue movements in living, unperturbed embryos. We label cells with a ubiquitously expressed transgene that encodes GFP fused to an autonomously folding actin binding fragment from fly moesin. Second, we use a biomechanical approach to examine the distribution of stiffness/tension during dorsal closure by following the response of the various tissues to cutting by an ultraviolet laser. We tested our previous model (Young, P.E., A.M. Richman, A.S. Ketchum, and D.P. Kiehart. 1993. Genes Dev. 7:29-41) that the leading edge of the lateral epidermis is a contractile purse-string that provides force for dorsal closure. We show that this structure is under tension and behaves as a supracellular purse-string, however, we provide evidence that it alone cannot account for the forces responsible for dorsal closure. In addition, we show that there is isotropic stiffness/tension in the amnioserosa and anisotropic stiffness/tension in the lateral epidermis. Tension in the amnioserosa may contribute force for dorsal closure, but tension in the lateral epidermis opposes it. Third, we examine the role of various tissues in dorsal closure by repeated ablation of cells in the amnioserosa and the leading edge of the lateral epidermis. Our data provide strong evidence that both tissues appear to contribute to normal dorsal closure in living embryos, but surprisingly, neither is absolutely required for dorsal closure. Finally, we establish that the Drosophila epidermis rapidly and reproducibly heals from both mechanical and ultraviolet laser wounds, even those delivered repeatedly. During healing, actin is rapidly recruited to the margins of the wound and a newly formed, supracellular purse-string contracts during wound healing. This result establishes the Drosophila embryo as an excellent system for the investigation of wound healing. Moreover, our observations demonstrate that wound healing in this insect epidermal system parallel wound healing in vertebrate tissues in situ and vertebrate cells in culture (for review see Kiehart, D.P. 1999. Curr. Biol. 9:R602-R605).
Figures


Similar articles
-
Nonmuscle myosin II generates forces that transmit tension and drive contraction in multiple tissues during dorsal closure.Curr Biol. 2005 Dec 20;15(24):2208-21. doi: 10.1016/j.cub.2005.11.064. Curr Biol. 2005. PMID: 16360683
-
Quantifying dorsal closure in three dimensions.Mol Biol Cell. 2016 Dec 15;27(25):3948-3955. doi: 10.1091/mbc.E16-06-0400. Epub 2016 Oct 26. Mol Biol Cell. 2016. PMID: 27798232 Free PMC article.
-
GFP-moesin illuminates actin cytoskeleton dynamics in living tissue and demonstrates cell shape changes during morphogenesis in Drosophila.Dev Biol. 1997 Nov 1;191(1):103-17. doi: 10.1006/dbio.1997.8707. Dev Biol. 1997. PMID: 9356175
-
[Dorsal closure in Drosophila. A genetic model for wound healing?].C R Acad Sci III. 1999 Jan;322(1):5-13. doi: 10.1016/s0764-4469(99)80012-2. C R Acad Sci III. 1999. PMID: 10047950 Review. French.
-
Cell Sheet Morphogenesis: Dorsal Closure in Drosophila melanogaster as a Model System.Annu Rev Cell Dev Biol. 2017 Oct 6;33:169-202. doi: 10.1146/annurev-cellbio-111315-125357. Annu Rev Cell Dev Biol. 2017. PMID: 28992442 Free PMC article. Review.
Cited by
-
Identifying Key Genetic Regions for Cell Sheet Morphogenesis on Chromosome 2L Using a Drosophila Deficiency Screen in Dorsal Closure.G3 (Bethesda). 2020 Nov 5;10(11):4249-4269. doi: 10.1534/g3.120.401386. G3 (Bethesda). 2020. PMID: 32978263 Free PMC article.
-
The actin cable is dispensable in directing dorsal closure dynamics but neutralizes mechanical stress to prevent scarring in the Drosophila embryo.Nat Cell Biol. 2016 Nov;18(11):1149-1160. doi: 10.1038/ncb3421. Epub 2016 Oct 17. Nat Cell Biol. 2016. PMID: 27749820
-
Laser-mediated cell ablation during post-implantation mouse development.Dev Dyn. 2013 Oct;242(10):1202-9. doi: 10.1002/dvdy.24017. Epub 2013 Sep 2. Dev Dyn. 2013. PMID: 23873840 Free PMC article.
-
A DPP-mediated feed-forward loop canalizes morphogenesis during Drosophila dorsal closure.J Cell Biol. 2015 Jan 19;208(2):239-48. doi: 10.1083/jcb.201410042. J Cell Biol. 2015. PMID: 25601405 Free PMC article.
-
Computational models for mechanics of morphogenesis.Birth Defects Res C Embryo Today. 2012 Jun;96(2):132-52. doi: 10.1002/bdrc.21013. Birth Defects Res C Embryo Today. 2012. PMID: 22692887 Free PMC article. Review.
References
-
- Agnes F., Noselli S. La fermeture dorsale chez la Drosophile. Un modèle génétique del cicatrisation? (Dorsal closure in Drosophila. A genetic model for wound healing?) C.R. Acad. Sci. Paris, Sciences de la vie. 1999;322:5–13. - PubMed
-
- Beloussov L.V., Kazakova N.I., Luchinskaia N.N., Novoselov V.V. Studies in developmental cytomechanic. Intl. J. Dev. Biol. 1997;41:793–799. - PubMed
-
- Bement W.M., Mandato C., Kirsch M.N. Wound-induced assembly and closure of an actomyosin purse string in Xenopus oocytes. Curr. Biol. 1999;9:579–587. - PubMed
-
- Brand A.H., Manoukian A.S., Perrimon N. Ectopic expression in Drosophila . In: Goldstein L.S.B., Fyrberg E.A., editors. Drosophila melanogaster: Practical Uses in Cell and Molecular Biology. Academic Press; San Diego, CA: 1994. pp. 635–654. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

