Attenuation of and protection induced by a leucine auxotroph of Mycobacterium tuberculosis
- PMID: 10768986
- PMCID: PMC97501
- DOI: 10.1128/IAI.68.5.2888-2898.2000
Attenuation of and protection induced by a leucine auxotroph of Mycobacterium tuberculosis
Abstract
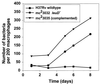
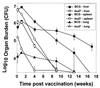
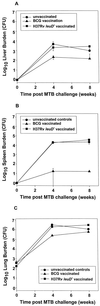
Attenuated mutants of Mycobacterium tuberculosis represent potential vaccine candidates for the prevention of tuberculosis. It is known that auxotrophs of a variety of bacteria are attenuated in vivo and yet provide protection against challenge with wild-type organisms. A leucine auxotroph of M. tuberculosis was created by allelic exchange, replacing wild-type leuD (Rv2987c), encoding isopropyl malate isomerase, with a mutant copy of the gene in which 359 bp had been deleted, creating a strain requiring exogenous leucine supplementation for growth in vitro. The frequency of reversion to prototrophy was <10(-11). In contrast to wild-type M. tuberculosis, the DeltaleuD mutant was unable to replicate in macrophages in vitro. Its attenuation in vivo and safety as a vaccine were established by the fact that it caused no deaths in immunodeficient SCID mice. Complementation of the mutant with wild-type leuD abolished the requirement for leucine supplementation and restored the ability of the strain to grow both in macrophages and in SCID mice, thus confirming that the attenuated phenotype was due to the DeltaleuD mutation. As a test of the vaccine potential of the leucine auxotroph, immunocompetent BALB/c mice, susceptible to fatal infection with wild-type M. tuberculosis, were immunized with the DeltaleuD mutant and subsequently challenged with virulent M. tuberculosis by both the intravenous and aerosol routes. A comparison group of mice was immunized with conventional Mycobacterium bovis BCG vaccine. Whereas all unvaccinated mice succumbed to intravenous infection within 15 weeks, mice immunized with either BCG or the DeltaleuD mutant of M. tuberculosis exhibited enhanced and statistically equivalent survival curves. However, the leuD auxotroph was less effective than live BCG in reducing organ burdens and tissue pathology of mice challenged by either route. We conclude that attenuation and protection against M. tuberculosis challenge can be achieved with a leucine auxotroph and suggest that to induce optimal protection, attenuated strains of M. tuberculosis should persist long enough and be sufficiently metabolically active to synthesize relevant antigens for an extended period of time.
Figures





Similar articles
-
Mycobacterium bovis DeltaleuD auxotroph-induced protective immunity against tissue colonization, burden and distribution in cattle intranasally challenged with Mycobacterium bovis Ravenel S.Vaccine. 2007 Feb 26;25(10):1743-55. doi: 10.1016/j.vaccine.2006.11.036. Epub 2006 Dec 6. Vaccine. 2007. PMID: 17240005
-
Persistence and protective efficacy of a Mycobacterium tuberculosis auxotroph vaccine.Infect Immun. 1999 Jun;67(6):2867-73. doi: 10.1128/IAI.67.6.2867-2873.1999. Infect Immun. 1999. PMID: 10338493 Free PMC article.
-
A pantothenate auxotroph of Mycobacterium tuberculosis is highly attenuated and protects mice against tuberculosis.Nat Med. 2002 Oct;8(10):1171-4. doi: 10.1038/nm765. Epub 2002 Sep 9. Nat Med. 2002. PMID: 12219086
-
Extended safety and efficacy studies of a live attenuated double leucine and pantothenate auxotroph of Mycobacterium tuberculosis as a vaccine candidate.Vaccine. 2011 Jun 24;29(29-30):4839-47. doi: 10.1016/j.vaccine.2011.04.066. Epub 2011 May 5. Vaccine. 2011. PMID: 21549795 Free PMC article.
-
[Novel vaccines against M. tuberculosis].Kekkaku. 2006 Dec;81(12):745-51. Kekkaku. 2006. PMID: 17240920 Review. Japanese.
Cited by
-
High throughput phenotypic analysis of Mycobacterium tuberculosis and Mycobacterium bovis strains' metabolism using biolog phenotype microarrays.PLoS One. 2013;8(1):e52673. doi: 10.1371/journal.pone.0052673. Epub 2013 Jan 10. PLoS One. 2013. PMID: 23326347 Free PMC article.
-
Chemical Mechanism of the Branched-Chain Aminotransferase IlvE from Mycobacterium tuberculosis.Biochemistry. 2016 Nov 15;55(45):6295-6303. doi: 10.1021/acs.biochem.6b00928. Epub 2016 Nov 2. Biochemistry. 2016. PMID: 27780341 Free PMC article.
-
Genome-wide requirements for Mycobacterium tuberculosis adaptation and survival in macrophages.Proc Natl Acad Sci U S A. 2005 Jun 7;102(23):8327-32. doi: 10.1073/pnas.0503272102. Epub 2005 May 31. Proc Natl Acad Sci U S A. 2005. PMID: 15928073 Free PMC article.
-
Brucella abortus Depends on l-Serine Biosynthesis for Intracellular Proliferation.Infect Immun. 2020 Jan 22;88(2):e00840-19. doi: 10.1128/IAI.00840-19. Print 2020 Jan 22. Infect Immun. 2020. PMID: 31740531 Free PMC article.
-
A novel biosafety level 2 compliant tuberculosis infection model using a ΔleuDΔpanCD double auxotroph of Mycobacterium tuberculosis H37Rv and Galleria mellonella.Virulence. 2020 Dec;11(1):811-824. doi: 10.1080/21505594.2020.1781486. Virulence. 2020. PMID: 32530737 Free PMC article.
References
-
- Ahmed Z U, Sarker M R, Sack D A. Protection of adult rabbits and monkeys from a lethal shigellosis by oral immunization with a thymine-requiring and temperature-sensitive mutant of Shigella flexneri Y. Vaccine. 1990;8:153–158. - PubMed
-
- Aronson J D, Aronson C F, Taylor H C. A twenty-year appraisal of BCG vaccination in the control of tuberculosis. Arch Intern Med. 1958;101:881–893. - PubMed
-
- Azad A K, Sirakova T D, Fernandes N D, Kolattukudy P E. Gene knockout reveals a novel gene cluster unique to pathogenic mycobacteria. J Biol Chem. 1997;272:16741–16745. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

