SUMO-1 conjugation to topoisomerase I: A possible repair response to topoisomerase-mediated DNA damage
- PMID: 10759568
- PMCID: PMC18143
- DOI: 10.1073/pnas.080536597
SUMO-1 conjugation to topoisomerase I: A possible repair response to topoisomerase-mediated DNA damage
Abstract
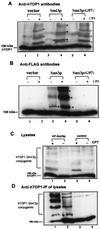
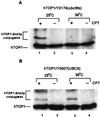
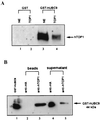
Ubiquitin/26S proteasome-dependent degradation of topoisomerase I (TOP1) has been suggested to be a unique repair response to TOP1-mediated DNA damage. In the current study, we show that treatment of mammalian cells or yeast cells expressing human DNA TOP1 with camptothecin (CPT) induces covalent modification of the TOP1 by SUMO-1/Smt3p, a ubiquitin-like protein. This conclusion is based on the following observations: (i) Mammalian DNA TOP1 conjugates induced by CPT were cross-reactive with SUMO-1/Smt3p-specific antibodies both in yeast expressing human DNA TOP1 as well as mammalian cells. (ii) The formation of TOP1 conjugates was shown to be dependent on UBC9, the E2 enzyme for SUMO-1/Smt3p. (iii) TOP1 physically interacts with UBC9. (iv) Ubc9 mutant yeast cells expressing human DNA TOP1 was hypersensitive to CPT, suggesting that UBC9/SUMO-1 may be involved in the repair of TOP1-mediated DNA damage.
Figures


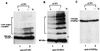
Similar articles
-
The ubiquitin-like proteins SMT3 and SUMO-1 are conjugated by the UBC9 E2 enzyme.Proc Natl Acad Sci U S A. 1998 Jan 20;95(2):560-4. doi: 10.1073/pnas.95.2.560. Proc Natl Acad Sci U S A. 1998. PMID: 9435231 Free PMC article.
-
Topoisomerase I-dependent viability loss in saccharomyces cerevisiae mutants defective in both SUMO conjugation and DNA repair.Genetics. 2007 Sep;177(1):17-30. doi: 10.1534/genetics.107.074708. Epub 2007 Jul 1. Genetics. 2007. PMID: 17603101 Free PMC article.
-
Defects in SUMO (small ubiquitin-related modifier) conjugation and deconjugation alter cell sensitivity to DNA topoisomerase I-induced DNA damage.J Biol Chem. 2005 Jun 24;280(25):23566-75. doi: 10.1074/jbc.M500947200. Epub 2005 Apr 6. J Biol Chem. 2005. PMID: 15817450
-
Topoisomerase degradation, DSB repair, p53 and IAPs in cancer cell resistance to camptothecin-like topoisomerase I inhibitors.Biochim Biophys Acta. 2013 Jan;1835(1):11-27. doi: 10.1016/j.bbcan.2012.09.002. Epub 2012 Sep 21. Biochim Biophys Acta. 2013. PMID: 23006513 Review.
-
Mechanisms of DNA topoisomerase I-induced cell killing in the yeast Saccharomyces cerevisiae.Ann N Y Acad Sci. 2000;922:65-75. doi: 10.1111/j.1749-6632.2000.tb07026.x. Ann N Y Acad Sci. 2000. PMID: 11193926 Review.
Cited by
-
ATM deficiency results in accumulation of DNA-topoisomerase I covalent intermediates in neural cells.PLoS One. 2013 Apr 23;8(4):e58239. doi: 10.1371/journal.pone.0058239. Print 2013. PLoS One. 2013. PMID: 23626666 Free PMC article.
-
Targets and consequences of protein SUMOylation in neurons.Brain Res Rev. 2010 Sep;64(1):195-212. doi: 10.1016/j.brainresrev.2010.04.002. Epub 2010 Apr 9. Brain Res Rev. 2010. PMID: 20382182 Free PMC article. Review.
-
SUMOylation of DISC1: a potential role in neural progenitor proliferation in the developing cortex.Mol Neuropsychiatry. 2016 May;2(1):20-27. doi: 10.1159/000444257. Epub 2016 Mar 15. Mol Neuropsychiatry. 2016. PMID: 27525255 Free PMC article.
-
Sumoylation modulates transcriptional activity of MITF in a promoter-specific manner.Pigment Cell Res. 2005 Aug;18(4):265-77. doi: 10.1111/j.1600-0749.2005.00234.x. Pigment Cell Res. 2005. PMID: 16029420 Free PMC article.
-
Topoisomerases and cancer chemotherapy: recent advances and unanswered questions.F1000Res. 2019 Sep 30;8:F1000 Faculty Rev-1704. doi: 10.12688/f1000research.20201.1. eCollection 2019. F1000Res. 2019. PMID: 31602296 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

