Envelope glycoprotein determinants of increased fusogenicity in a pathogenic simian-human immunodeficiency virus (SHIV-KB9) passaged in vivo
- PMID: 10756060
- PMCID: PMC111962
- DOI: 10.1128/jvi.74.9.4433-4440.2000
Envelope glycoprotein determinants of increased fusogenicity in a pathogenic simian-human immunodeficiency virus (SHIV-KB9) passaged in vivo
Abstract
Changes in the envelope glycoprotein ectodomains of a nonpathogenic simian-human immunodeficiency virus (SHIV-89.6) that was serially passaged in vivo have been shown to be responsible for the increased pathogenicity of the resulting virus, SHIV-KB9 (G. B. Karlsson, et al., J. Exp. Med. 188:1159-1171, 1998). The 12 amino acid changes in the envelope glycoprotein ectodomains resulted in increased chemokine receptor-binding and syncytium-forming abilities. Here we identify the envelope glycoprotein determinants of these properties. A single amino acid change in the gp120 third variable (V3) loop was both necessary and sufficient for the observed increase in the binding of the SHIV-KB9 gp120 glycoprotein to the CCR5 chemokine receptor. The increased syncytium-forming ability of SHIV-KB9 involved, in addition to the V3 loop change, changes in the second conserved (C2) region of gp120 (residue 225) and in the gp41 ectodomain (residues 564 and 567). The C2 and gp41 ectodomain changes influenced syncytium formation in a cooperative manner. Changes in the V1/V2 gp120 variable loops exerted a negative effect on syncytium formation and chemokine receptor binding, supporting a previously described role of these changes in immune evasion. The definition of the passage-associated changes that determine the efficiency of chemokine receptor binding and membrane fusogenicity will allow evaluation of the contribution of these properties to in vivo CD4-positive lymphocyte depletion.
Figures

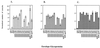
Similar articles
-
Membrane-fusing capacity of the human immunodeficiency virus envelope proteins determines the efficiency of CD+ T-cell depletion in macaques infected by a simian-human immunodeficiency virus.J Virol. 2001 Jun;75(12):5646-55. doi: 10.1128/JVI.75.12.5646-5655.2001. J Virol. 2001. PMID: 11356972 Free PMC article.
-
Envelope glycoprotein determinants of increased entry in a pathogenic simian-human immunodeficiency virus (SHIV-HXBc2P 3.2) passaged in monkeys.AIDS Res Hum Retroviruses. 2004 Feb;20(2):163-73. doi: 10.1089/088922204773004888. AIDS Res Hum Retroviruses. 2004. PMID: 15018704
-
Induction of antibodies in guinea pigs and rhesus monkeys against the human immunodeficiency virus type 1 envelope: neutralization of nonpathogenic and pathogenic primary isolate simian/human immunodeficiency virus strains.J Virol. 2000 Jan;74(1):254-63. doi: 10.1128/jvi.74.1.254-263.2000. J Virol. 2000. PMID: 10590113 Free PMC article.
-
Understanding the basis of CD4(+) T-cell depletion in macaques infected by a simian-human immunodeficiency virus.Vaccine. 2002 May 6;20(15):1934-7. doi: 10.1016/s0264-410x(02)00072-5. Vaccine. 2002. PMID: 11983249 Review.
-
HIV entry inhibitors: mechanisms of action and resistance pathways.J Antimicrob Chemother. 2006 Apr;57(4):619-27. doi: 10.1093/jac/dkl027. Epub 2006 Feb 7. J Antimicrob Chemother. 2006. PMID: 16464888 Review.
Cited by
-
Longitudinal Analysis of CCR5 and CXCR4 Usage in a Cohort of Antiretroviral Therapy-Naïve Subjects with Progressive HIV-1 Subtype C Infection.PLoS One. 2013 Jun 18;8(6):e65950. doi: 10.1371/journal.pone.0065950. Print 2013. PLoS One. 2013. PMID: 23824043 Free PMC article.
-
The HR2 polymorphism N140I in the HIV-1 gp41 combined with the HR1 V38A mutation is associated with a less cytopathic phenotype.Retrovirology. 2012 Feb 14;9:15. doi: 10.1186/1742-4690-9-15. Retrovirology. 2012. PMID: 22333046 Free PMC article.
-
Importance of membrane fusion mediated by human immunodeficiency virus envelope glycoproteins for lysis of primary CD4-positive T cells.J Virol. 2000 Nov;74(22):10690-8. doi: 10.1128/jvi.74.22.10690-10698.2000. J Virol. 2000. PMID: 11044113 Free PMC article.
-
Macrophage tropism of human immunodeficiency virus type 1 isolates from brain and lymphoid tissues predicts neurotropism independent of coreceptor specificity.J Virol. 2001 Nov;75(21):10073-89. doi: 10.1128/JVI.75.21.10073-10089.2001. J Virol. 2001. PMID: 11581376 Free PMC article.
-
Linkage of reduced receptor affinity and superinfection to pathogenesis of TR1.3 murine leukemia virus.J Virol. 2006 May;80(9):4601-9. doi: 10.1128/JVI.80.9.4601-4609.2006. J Virol. 2006. PMID: 16611920 Free PMC article.
References
-
- Alkhatib G, Combadiere C, Broder C C, Feng Y, Kennedy P E, Murphy P M, Berger E A. CC CKR5: a RANTES, MIP-1alpha, MIP-1beta receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1958. - PubMed
-
- Barre-Sinoussi F, Chermann J C, Rey F, Nugeyre M T, Chamaret S, Gruest J, Dauguet C, Axler-Blin C, Vezinet-Brun F, Rouzioux C, Rozenbaum W, Montagnier L. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS) Science. 1983;220:868–871. - PubMed
-
- Chan D C, Fass D, Berger J M, Kim P S. Core structure of gp41 from the HIV envelope glycoprotein. Cell. 1997;89:263–273. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

