Cardiac fibrosis in mice lacking brain natriuretic peptide
- PMID: 10737768
- PMCID: PMC18212
- DOI: 10.1073/pnas.070371497
Cardiac fibrosis in mice lacking brain natriuretic peptide
Abstract
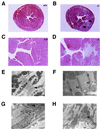
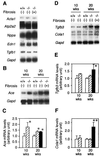
Cardiac fibrosis, defined as a proliferation of interstitial fibroblasts and biosynthesis of extracellular matrix components in the ventricles of the heart, is a consequence of remodeling processes initiated by pathologic events associated with a variety of cardiovascular disorders, which leads to abnormal myocardial stiffness and, ultimately, ventricular dysfunction. Brain natriuretic peptide (BNP) is a cardiac hormone produced primarily by ventricular myocytes, and its plasma concentrations are markedly elevated in patients with congestive heart failure and acute myocardial infarction. However, its precise functional significance has been undefined. In this paper, we report the generation of mice with targeted disruption of BNP (Nppb(-/-) mice). We observed multifocal fibrotic lesions in the ventricles from Nppb(-/-) mice. No signs of systemic hypertension and ventricular hypertrophy are noted in Nppb(-/-) mice. In response to ventricular pressure overload, focal fibrotic lesions are increased in size and number in Nppb(-/-) mice, whereas no focal fibrotic changes are found in wild-type littermates (Nppb(+/+) mice). This study establishes BNP as a cardiomyocyte-derived antifibrotic factor in vivo and provides evidence for its role as a local regulator of ventricular remodeling.
Figures


Similar articles
-
Brain natriuretic peptide appears to act locally as an antifibrotic factor in the heart.Can J Physiol Pharmacol. 2001 Aug;79(8):723-9. Can J Physiol Pharmacol. 2001. PMID: 11558681
-
Intramyocardial BNP gene delivery improves cardiac function through distinct context-dependent mechanisms.Circ Heart Fail. 2011 Jul;4(4):483-95. doi: 10.1161/CIRCHEARTFAILURE.110.958033. Epub 2011 May 10. Circ Heart Fail. 2011. PMID: 21558448
-
B-Type Natriuretic Peptide Deletion Leads to Progressive Hypertension, Associated Organ Damage, and Reduced Survival: Novel Model for Human Hypertension.Hypertension. 2015 Jul;66(1):199-210. doi: 10.1161/HYPERTENSIONAHA.115.05610. Epub 2015 May 11. Hypertension. 2015. PMID: 26063669 Free PMC article.
-
Mechanical load-induced alterations in B-type natriuretic peptide gene expression.Can J Physiol Pharmacol. 2001 Aug;79(8):646-53. Can J Physiol Pharmacol. 2001. PMID: 11558673 Review.
-
The role of natriuretic peptides in cardioprotection.Cardiovasc Res. 2006 Feb 1;69(2):318-28. doi: 10.1016/j.cardiores.2005.10.001. Epub 2005 Nov 10. Cardiovasc Res. 2006. PMID: 16289003 Review.
Cited by
-
Biomarkers in the evaluation of cardiac involvement in systemic sclerosis.Rheumatol Immunol Res. 2024 Jul 15;5(2):99-106. doi: 10.1515/rir-2024-0013. eCollection 2024 Jun. Rheumatol Immunol Res. 2024. PMID: 39015844 Free PMC article.
-
Targeting cardiac fibrosis in heart failure with preserved ejection fraction: mirage or miracle?EMBO Mol Med. 2020 Oct 7;12(10):e10865. doi: 10.15252/emmm.201910865. Epub 2020 Sep 21. EMBO Mol Med. 2020. PMID: 32955172 Free PMC article. Review.
-
Lethal arrhythmias in Tbx3-deficient mice reveal extreme dosage sensitivity of cardiac conduction system function and homeostasis.Proc Natl Acad Sci U S A. 2012 Jan 17;109(3):E154-63. doi: 10.1073/pnas.1115165109. Epub 2011 Dec 27. Proc Natl Acad Sci U S A. 2012. PMID: 22203979 Free PMC article.
-
A novel variable number of tandem repeat of the natriuretic peptide precursor B gene's 5'-flanking region is associated with essential hypertension among Japanese females.Int J Med Sci. 2007 May 16;4(3):146-52. doi: 10.7150/ijms.4.146. Int J Med Sci. 2007. PMID: 17554401 Free PMC article.
-
Cardiovascular Pleiotropic Effects of Natriuretic Peptides.Int J Mol Sci. 2019 Aug 8;20(16):3874. doi: 10.3390/ijms20163874. Int J Mol Sci. 2019. PMID: 31398927 Free PMC article. Review.
References
-
- Weber K T, Brilla C G. Circulation. 1991;83:1849–1865. - PubMed
-
- Butt R P, Laurent G J, Bishop J E. Ann NY Acad Sci. 1995;752:387–393. - PubMed
-
- Sudoh T, Kangawa K, Minamino N, Matsuo H. Nature (London) 1988;332:78–81. - PubMed
-
- Ogawa Y, Nakao K. In: Hypertension: Pathophysiology, Diagnosis, and Management. Laragh J H, Brenner B M, editors. New York: Raven; 1995. pp. 833–840.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

