The formation of immunogenic major histocompatibility complex class II-peptide ligands in lysosomal compartments of dendritic cells is regulated by inflammatory stimuli
- PMID: 10727455
- PMCID: PMC2193115
- DOI: 10.1084/jem.191.6.927
The formation of immunogenic major histocompatibility complex class II-peptide ligands in lysosomal compartments of dendritic cells is regulated by inflammatory stimuli
Abstract
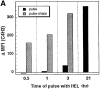
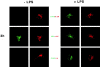
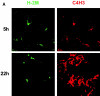
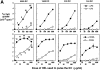
During their final differentiation or maturation, dendritic cells (DCs) redistribute their major histocompatibility complex (MHC) class II products from intracellular compartments to the plasma membrane. Using cells arrested in the immature state, we now find that DCs also regulate the initial intracellular formation of immunogenic MHC class II-peptide complexes. Immature DCs internalize the protein antigen, hen egg lysozyme (HEL), into late endosomes and lysosomes rich in MHC class II molecules. There, despite extensive colocalization of HEL protein and MHC class II products, MHC class II-peptide complexes do not form unless the DCs are exposed to inflammatory mediators such as tumor necrosis factor alpha, CD40 ligand, or lipoplolysaccharide. The control of T cell receptor (TCR) ligand formation was observed using the C4H3 monoclonal antibody to detect MHC class II-HEL peptide complexes by flow cytometry and confocal microscopy, and with HEL-specific 3A9 transgenic T cells to detect downregulation of the TCR upon MHC-peptide encounter. Even the binding of preprocessed HEL peptide to MHC class II is blocked in immature DCs, including the formation of C4H3 epitope in MHC class II compartments, suggesting an arrest to antigen presentation at the peptide-loading step, rather than an enhanced degradation of MHC class II-peptide complexes at the cell surface, as described in previous work. Therefore, the capacity of late endosomes and lysosomes to produce MHC class II-peptide complexes can be strictly controlled during DC differentiation, helping to coordinate antigen acquisition and inflammatory stimuli with formation of TCR ligands. The increased ability of maturing DCs to load MHC class II molecules with antigenic cargo contributes to the >100-fold enhancement of the subsequent primary immune response observed when immature and mature DCs are compared as immune adjuvants in culture and in mice.
Figures






Similar articles
-
Transport of peptide-MHC class II complexes in developing dendritic cells.Science. 2000 Apr 21;288(5465):522-7. doi: 10.1126/science.288.5465.522. Science. 2000. PMID: 10775112
-
CpG DNA induces maturation of dendritic cells with distinct effects on nascent and recycling MHC-II antigen-processing mechanisms.J Immunol. 2000 Dec 15;165(12):6889-95. doi: 10.4049/jimmunol.165.12.6889. J Immunol. 2000. PMID: 11120813
-
Antigen-unspecific B cells and lymphoid dendritic cells both show extensive surface expression of processed antigen-major histocompatibility complex class II complexes after soluble protein exposure in vivo or in vitro.J Exp Med. 1997 Aug 29;186(5):673-82. doi: 10.1084/jem.186.5.673. J Exp Med. 1997. PMID: 9271583 Free PMC article.
-
Control of MHC class II antigen presentation in dendritic cells: a balance between creative and destructive forces.Immunol Rev. 2005 Oct;207:191-205. doi: 10.1111/j.0105-2896.2005.00317.x. Immunol Rev. 2005. PMID: 16181337 Review.
-
Endosomal sorting of MHC class II determines antigen presentation by dendritic cells.Curr Opin Cell Biol. 2008 Aug;20(4):437-44. doi: 10.1016/j.ceb.2008.05.011. Epub 2008 Jul 5. Curr Opin Cell Biol. 2008. PMID: 18582577 Review.
Cited by
-
Assessment of immunotoxicity using precision-cut tissue slices.Xenobiotica. 2013 Jan;43(1):84-97. doi: 10.3109/00498254.2012.731543. Epub 2012 Nov 16. Xenobiotica. 2013. PMID: 23199366 Free PMC article. Review.
-
Disruption of multivesicular body vesicles does not affect major histocompatibility complex (MHC) class II-peptide complex formation and antigen presentation by dendritic cells.J Biol Chem. 2013 Aug 23;288(34):24286-92. doi: 10.1074/jbc.M113.461996. Epub 2013 Jul 11. J Biol Chem. 2013. PMID: 23846690 Free PMC article.
-
Interleukin (IL)-4 inhibits IL-10 to promote IL-12 production by dendritic cells.J Exp Med. 2005 Jun 20;201(12):1899-903. doi: 10.1084/jem.20050324. J Exp Med. 2005. PMID: 15967820 Free PMC article.
-
Avoiding horror autotoxicus: the importance of dendritic cells in peripheral T cell tolerance.Proc Natl Acad Sci U S A. 2002 Jan 8;99(1):351-8. doi: 10.1073/pnas.231606698. Epub 2002 Jan 2. Proc Natl Acad Sci U S A. 2002. PMID: 11773639 Free PMC article. Review.
-
The linkage of innate to adaptive immunity via maturing dendritic cells in vivo requires CD40 ligation in addition to antigen presentation and CD80/86 costimulation.J Exp Med. 2004 Jun 21;199(12):1607-18. doi: 10.1084/jem.20040317. Epub 2004 Jun 14. J Exp Med. 2004. PMID: 15197224 Free PMC article.
References
-
- Hart D.N. Dendritic cellsunique leukocyte populations which control the primary immune response. Blood. 1997;90:3245–3287. - PubMed
-
- Banchereau J., Steinman R.M. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. - PubMed
-
- Romani N., Koide S., Crowley M., Witmer-Pack M., Livingstone A.M., Fathman C.G., Inaba K., Steinman R.M. Presentation of exogenous protein antigens by dendritic cells to T cell clonesintact protein is presented best by immature, epidermal Langerhans cells. J. Exp. Med. 1989;169:1169–1178. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

