Identification and localization of two brefeldin A-inhibited guanine nucleotide-exchange proteins for ADP-ribosylation factors in a macromolecular complex
- PMID: 10716990
- PMCID: PMC15969
- DOI: 10.1073/pnas.97.6.2567
Identification and localization of two brefeldin A-inhibited guanine nucleotide-exchange proteins for ADP-ribosylation factors in a macromolecular complex
Abstract
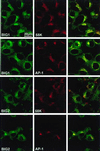
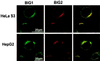
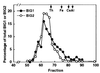
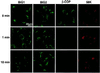
Two brefeldin A (BFA)-inhibited guanine nucleotide-exchange proteins for ADP-ribosylation factors, 200-kDa BIG1 and 190-kDa BIG2, were copurified from bovine brain cytosol associated with >670-kDa macromolecular complexes. When observed by immunofluorescence in HeLa S3 and HepG2 cells, endogenous BIG1 and coexpressed BIG2 were distributed in a punctate pattern throughout the cytosol, and also concentrated in the perinuclear region, where endogenous BIG1 and BIG2 each partially colocalized with Golgi-specific 58K protein and gamma-adaptin. On Western blot analysis, both BIG1 and BIG2 were clearly more abundant in the cytosol than in the microsomal fractions. After density gradient centrifugation of a microsomal fraction, BIG1 and BIG2 were recovered in the same fraction as beta-COP, a marker for Golgi membranes. When cytosol from HeLa S3 cells was subjected to gel filtration and fractions were analyzed by Western blotting, the largest percentages of both BIG1 and BIG2 were detected in fractions containing proteins with a molecular mass of >670 kDa. Western blotting using anti-peptide antibodies specific for BIG1 or BIG2 demonstrated that approximately 70% of BIG2 was immunoprecipitated along with 100% of BIG1 by the anti-BIG1 IgG, and approximately 75% of BIG1 was coprecipitated with 100% of BIG2 by the anti-BIG2 IgG. All observations were consistent with the conclusion that significant fractions of BIG1 and BIG2 exist as components of the same macromolecular complexes in bovine brain cytosol and are similarly localized in cultured cells.
Figures




Similar articles
-
Nuclear localization and molecular partners of BIG1, a brefeldin A-inhibited guanine nucleotide-exchange protein for ADP-ribosylation factors.Proc Natl Acad Sci U S A. 2004 Mar 2;101(9):2752-7. doi: 10.1073/pnas.0307345101. Epub 2004 Feb 18. Proc Natl Acad Sci U S A. 2004. PMID: 14973189 Free PMC article.
-
Purification and cloning of a brefeldin A-inhibited guanine nucleotide-exchange protein for ADP-ribosylation factors.J Biol Chem. 1999 Apr 30;274(18):12308-15. doi: 10.1074/jbc.274.18.12308. J Biol Chem. 1999. PMID: 10212200
-
Interaction of FK506-binding protein 13 with brefeldin A-inhibited guanine nucleotide-exchange protein 1 (BIG1): effects of FK506.Proc Natl Acad Sci U S A. 2003 Mar 4;100(5):2322-7. doi: 10.1073/pnas.2628047100. Epub 2003 Feb 26. Proc Natl Acad Sci U S A. 2003. PMID: 12606707 Free PMC article.
-
Arf, Sec7 and Brefeldin A: a model towards the therapeutic inhibition of guanine nucleotide-exchange factors.Biochem Soc Trans. 2005 Dec;33(Pt 6):1265-8. doi: 10.1042/BST0331265. Biochem Soc Trans. 2005. PMID: 16246094 Review.
-
Activation of toxin ADP-ribosyltransferases by eukaryotic ADP-ribosylation factors.Mol Cell Biochem. 1999 Mar;193(1-2):153-7. Mol Cell Biochem. 1999. PMID: 10331652 Review.
Cited by
-
BIG1, a brefeldin A-inhibited guanine nucleotide-exchange protein modulates ATP-binding cassette transporter A-1 trafficking and function.Arterioscler Thromb Vasc Biol. 2013 Feb;33(2):e31-8. doi: 10.1161/ATVBAHA.112.300720. Epub 2012 Dec 6. Arterioscler Thromb Vasc Biol. 2013. PMID: 23220274 Free PMC article.
-
Association of guanine nucleotide-exchange protein BIG1 in HepG2 cell nuclei with nucleolin, U3 snoRNA, and fibrillarin.Proc Natl Acad Sci U S A. 2008 Mar 4;105(9):3357-61. doi: 10.1073/pnas.0712387105. Epub 2008 Feb 21. Proc Natl Acad Sci U S A. 2008. PMID: 18292223 Free PMC article.
-
Arf guanine nucleotide-exchange factors BIG1 and BIG2 regulate nonmuscle myosin IIA activity by anchoring myosin phosphatase complex.Proc Natl Acad Sci U S A. 2013 Aug 20;110(34):E3162-70. doi: 10.1073/pnas.1312531110. Epub 2013 Aug 5. Proc Natl Acad Sci U S A. 2013. PMID: 23918382 Free PMC article.
-
Localization of large ADP-ribosylation factor-guanine nucleotide exchange factors to different Golgi compartments: evidence for distinct functions in protein traffic.Mol Biol Cell. 2002 Jan;13(1):119-33. doi: 10.1091/mbc.01-08-0420. Mol Biol Cell. 2002. PMID: 11809827 Free PMC article.
-
Unfolded protein response and cell death after depletion of brefeldin A-inhibited guanine nucleotide-exchange protein GBF1.Proc Natl Acad Sci U S A. 2008 Feb 26;105(8):2877-82. doi: 10.1073/pnas.0712224105. Epub 2008 Feb 14. Proc Natl Acad Sci U S A. 2008. PMID: 18287014 Free PMC article.
References
-
- Rothman J E, Wieland F T. Science. 1996;272:227–234. - PubMed
-
- Springer S, Spang A, Schekman R. Cell. 1999;97:145–198. - PubMed
-
- Tsuchiya M, Price S R, Tsai S-C, Moss J, Vaughan M. J Biol Chem. 1991;266:2772–2777. - PubMed
-
- Palmer D J, Helms J B, Beckers C J, Orci L, Rothman J E. J Biol Chem. 1993;268:12083–12089. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

