Lipid rafts function in biosynthetic delivery of proteins to the cell surface in yeast
- PMID: 10716729
- PMCID: PMC16225
- DOI: 10.1073/pnas.97.7.3254
Lipid rafts function in biosynthetic delivery of proteins to the cell surface in yeast
Abstract
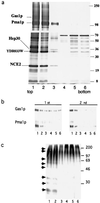
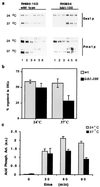
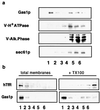
Lipid rafts, formed by lateral association of sphingolipids and cholesterol, have been implicated in membrane traffic and cell signaling in mammalian cells. Sphingolipids also have been shown to play a role in protein sorting in yeast. Therefore, we wanted to investigate whether lipid rafts exist in yeast and whether these membrane microdomains have an analogous function to their mammalian counterparts. We first developed a protocol for isolating detergent-insoluble glycolipid-enriched complexes (DIGs) from yeast cells. Sequencing of the major protein components of the isolated DIGs by mass spectrometry allowed us to identify, among others, Gas1p, Pma1p, and Nce2p. Using lipid biosynthetic mutants we could demonstrate that conditions that impair the synthesis of sphingolipids and ergosterol also disrupt raft association of Gas1p and Pma1p but not the secretion of acid phosphatase. That endoplasmic reticulum (ER)-to-Golgi transport of Gas1p is blocked in the sphingolipid mutant lcb1-100 raised the question of whether proteins associate with lipid rafts in the ER or later as shown in mammalian cells. Using the sec18-1 mutant we found that DIGs are present already in the ER. Taken together, our results suggest that lipid rafts are involved in the biosynthetic delivery of proteins to the yeast plasma membrane.
Figures



Similar articles
-
Synthesis of sphingolipids with very long chain fatty acids but not ergosterol is required for routing of newly synthesized plasma membrane ATPase to the cell surface of yeast.J Biol Chem. 2005 Jun 10;280(23):22515-22. doi: 10.1074/jbc.M413472200. Epub 2005 Apr 6. J Biol Chem. 2005. PMID: 15817474
-
A specific structural requirement for ergosterol in long-chain fatty acid synthesis mutants important for maintaining raft domains in yeast.Mol Biol Cell. 2002 Dec;13(12):4414-28. doi: 10.1091/mbc.e02-02-0116. Mol Biol Cell. 2002. PMID: 12475962 Free PMC article.
-
Lactoferrin perturbs lipid rafts and requires integrity of Pma1p-lipid rafts association to exert its antifungal activity against Saccharomyces cerevisiae.Int J Biol Macromol. 2021 Feb 28;171:343-357. doi: 10.1016/j.ijbiomac.2020.12.224. Epub 2021 Jan 7. Int J Biol Macromol. 2021. PMID: 33421469
-
The way we view cellular (glyco)sphingolipids.J Neurochem. 2007 Nov;103 Suppl 1:3-13. doi: 10.1111/j.1471-4159.2007.04721.x. J Neurochem. 2007. PMID: 17986134 Review.
-
Detergent-insoluble glycosphingolipid/cholesterol-rich membrane domains, lipid rafts and caveolae (review).Mol Membr Biol. 1999 Apr-Jun;16(2):145-56. doi: 10.1080/096876899294607. Mol Membr Biol. 1999. PMID: 10417979 Review.
Cited by
-
Partitioning into ER membrane microdomains impacts autophagic protein turnover during cellular aging.Sci Rep. 2024 Jun 13;14(1):13653. doi: 10.1038/s41598-024-64493-8. Sci Rep. 2024. PMID: 38871812 Free PMC article.
-
Sphingolipid diversity in Candida auris: unraveling interclade and drug resistance fingerprints.FEMS Yeast Res. 2024 Jan 9;24:foae008. doi: 10.1093/femsyr/foae008. FEMS Yeast Res. 2024. PMID: 38444195 Free PMC article.
-
Cellular Responses and Targets in Food Spoilage Yeasts Exposed to Antifungal Prenylated Isoflavonoids.Microbiol Spectr. 2023 Aug 17;11(4):e0132723. doi: 10.1128/spectrum.01327-23. Epub 2023 Jul 10. Microbiol Spectr. 2023. PMID: 37428107 Free PMC article.
-
Ergosterol distribution controls surface structure formation and fungal pathogenicity.mBio. 2023 Aug 31;14(4):e0135323. doi: 10.1128/mbio.01353-23. Epub 2023 Jul 6. mBio. 2023. PMID: 37409809 Free PMC article.
-
Ergosterol distribution controls surface structure formation and fungal pathogenicity.bioRxiv [Preprint]. 2023 Feb 17:2023.02.17.528979. doi: 10.1101/2023.02.17.528979. bioRxiv. 2023. Update in: mBio. 2023 Aug 31;14(4):e0135323. doi: 10.1128/mbio.01353-23. PMID: 36824733 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

