Tcl1 enhances Akt kinase activity and mediates its nuclear translocation
- PMID: 10716693
- PMCID: PMC16186
- DOI: 10.1073/pnas.97.7.3028
Tcl1 enhances Akt kinase activity and mediates its nuclear translocation
Abstract
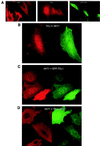
The TCL1 oncogene at 14q32.1 is involved in the development of human mature T-cell leukemia. The mechanism of action of Tcl1 is unknown. Because the virus containing the v-akt oncogene causes T-cell lymphoma in mice and Akt is a key player in transduction of antiapoptotic and proliferative signals in T-cells, we investigated whether Akt and Tcl1 function in the same pathway. Coimmunoprecipitation experiments showed that endogenous Akt1 and Tcl1 physically interact in the T-cell leukemia cell line SupT11; both proteins also interact when cotransfected into 293 cells. Using several AKT1 constructs in cotransfection experiments, we determined that this interaction occurs through the pleckstrin homology domain of the Akt1 protein. We further demonstrated that, in 293 cells transfected with TCL1, the endogenous Akt1 bound to Tcl1 is 5-10 times more active compared with Akt1 not bound to Tcl1. The intracellular localization of Tcl1 and Akt1 in mouse fibroblasts was investigated by immunofluorescence. When transfected alone, Akt1 was found only in cytoplasm whereas Tcl1 was localized in the cytoplasm and in the nucleus. Interestingly, Akt1 was also found in the nucleus when AKT1 was cotransfected with TCL1, suggesting that Tcl1 promotes the transport of Akt1 to the nucleus. These findings were supported by the intracellular localization of Akt1 or Tcl1 when Tcl1 or Akt1, respectively, were confined to the specific cellular compartments. Thus, we demonstrate that Tcl1 is a cofactor of Akt1 that enhances Akt1 kinase activity and promotes its nuclear transport.
Figures
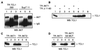
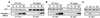


Similar articles
-
Differential regulation of Akt kinase isoforms by the members of the TCL1 oncogene family.J Biol Chem. 2002 Feb 1;277(5):3743-51. doi: 10.1074/jbc.M107069200. Epub 2001 Nov 13. J Biol Chem. 2002. PMID: 11707444
-
The role of TCL1 in human T-cell leukemia.Oncogene. 2001 Sep 10;20(40):5638-43. doi: 10.1038/sj.onc.1204596. Oncogene. 2001. PMID: 11607815 Review.
-
A modeled hydrophobic domain on the TCL1 oncoprotein mediates association with AKT at the cytoplasmic membrane.Biochemistry. 2002 May 21;41(20):6376-82. doi: 10.1021/bi016068o. Biochemistry. 2002. PMID: 12009899
-
Regulation of TCL1 expression in B- and T-cell lymphomas and reactive lymphoid tissues.Cancer Res. 2000 Apr 15;60(8):2095-100. Cancer Res. 2000. PMID: 10786666
-
Molecular basis of mature T-cell leukemia.JAMA. 2001 Nov 14;286(18):2308-14. doi: 10.1001/jama.286.18.2308. JAMA. 2001. PMID: 11710897 Review.
Cited by
-
TCL1A and ATM are co-expressed in chronic lymphocytic leukemia cells without deletion of 11q.Haematologica. 2013 Feb;98(2):269-73. doi: 10.3324/haematol.2012.070623. Epub 2012 Aug 8. Haematologica. 2013. PMID: 22875623 Free PMC article.
-
Noncoding RNA genes in cancer pathogenesis.Adv Biol Regul. 2019 Jan;71:219-223. doi: 10.1016/j.jbior.2018.12.002. Epub 2018 Dec 13. Adv Biol Regul. 2019. PMID: 30611710 Free PMC article. Review.
-
Interaction between TCL1 and Epac1 in the activation of Akt kinases in plasma membranes and nuclei of 8-CPT-2-O-Me-cAMP-stimulated macrophages.Cell Signal. 2008 Jan;20(1):130-8. doi: 10.1016/j.cellsig.2007.10.008. Epub 2007 Oct 12. Cell Signal. 2008. PMID: 17993260 Free PMC article.
-
MicroRNAs in thyroid cancer.J Clin Endocrinol Metab. 2011 Nov;96(11):3326-36. doi: 10.1210/jc.2011-1004. Epub 2011 Aug 24. J Clin Endocrinol Metab. 2011. PMID: 21865360 Free PMC article. Review.
-
Nuclear and mitochondrial signalling Akts in cardiomyocytes.Cardiovasc Res. 2009 May 1;82(2):272-85. doi: 10.1093/cvr/cvp087. Epub 2009 Mar 11. Cardiovasc Res. 2009. PMID: 19279164 Free PMC article. Review.
References
-
- Soulier J, Madani A, Cacheux V, Rosenzwajg M, Sigaux F, Stern M H. Oncogene. 1994;9:3565–3570. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

