Synthesis, biological activity, and molecular modeling of ribose-modified deoxyadenosine bisphosphate analogues as P2Y(1) receptor ligands
- PMID: 10715151
- PMCID: PMC9364911
- DOI: 10.1021/jm990249v
Synthesis, biological activity, and molecular modeling of ribose-modified deoxyadenosine bisphosphate analogues as P2Y(1) receptor ligands
Abstract
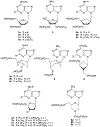
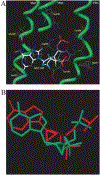
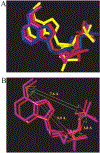
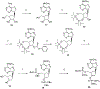
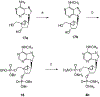
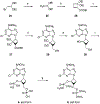
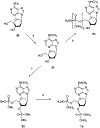
The structure-activity relationships of adenosine-3', 5'-bisphosphates as P2Y(1) receptor antagonists have been explored, revealing the potency-enhancing effects of the N(6)-methyl group and the ability to substitute the ribose moiety (Nandanan et al. J. Med. Chem. 1999, 42, 1625-1638). We have introduced constrained carbocyclic rings (to explore the role of sugar puckering), non-glycosyl bonds to the adenine moiety, and a phosphate group shift. The biological activity of each analogue at P2Y(1) receptors was characterized by measuring its capacity to stimulate phospholipase C in turkey erythrocyte membranes (agonist effect) and to inhibit its stimulation elicited by 30 nM 2-methylthioadenosine-5'-diphosphate (antagonist effect). Addition of the N(6)-methyl group in several cases converted pure agonists to antagonists. A carbocyclic N(6)-methyl-2'-deoxyadenosine bisphosphate analogue was a pure P2Y(1) receptor antagonist and equipotent to the ribose analogue (MRS 2179). In the series of ring-constrained methanocarba derivatives where a fused cyclopropane moiety constrained the pseudosugar ring of the nucleoside to either a Northern (N) or Southern (S) conformation, as defined in the pseudorotational cycle, the 6-NH(2) (N)-analogue was a pure agonist of EC(50) 155 nM and 86-fold more potent than the corresponding (S)-isomer. The 2-chloro-N(6)-methyl-(N)-methanocarba analogue was an antagonist of IC(50) 51.6 nM. Thus, the ribose ring (N)-conformation appeared to be favored in recognition at P2Y(1) receptors. A cyclobutyl analogue was an antagonist with IC(50) of 805 nM, while morpholine ring-containing analogues were nearly inactive. Anhydrohexitol ring-modified bisphosphate derivatives displayed micromolar potency as agonists (6-NH(2)) or antagonists (N(6)-methyl). A molecular model of the energy-minimized structures of the potent antagonists suggested that the two phosphate groups may occupy common regions. The (N)- and (S)-methanocarba agonist analogues were docked into the putative binding site of the previously reported P2Y(1) receptor model.
Figures


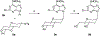

Similar articles
-
Adenine nucleotide analogues locked in a Northern methanocarba conformation: enhanced stability and potency as P2Y(1) receptor agonists.J Med Chem. 2002 May 9;45(10):2090-100. doi: 10.1021/jm010538v. J Med Chem. 2002. PMID: 11985476 Free PMC article.
-
Structure-activity relationships of bisphosphate nucleotide derivatives as P2Y1 receptor antagonists and partial agonists.J Med Chem. 1999 May 6;42(9):1625-38. doi: 10.1021/jm980657j. J Med Chem. 1999. PMID: 10229631 Free PMC article.
-
Methanocarba modification of uracil and adenine nucleotides: high potency of Northern ring conformation at P2Y1, P2Y2, P2Y4, and P2Y11 but not P2Y6 receptors.J Med Chem. 2002 Jan 3;45(1):208-18. doi: 10.1021/jm010369e. J Med Chem. 2002. PMID: 11754592 Free PMC article.
-
Development of selective high affinity antagonists, agonists, and radioligands for the P2Y1 receptor.Comb Chem High Throughput Screen. 2008 Jul;11(6):410-9. doi: 10.2174/138620708784911474. Comb Chem High Throughput Screen. 2008. PMID: 18673269 Free PMC article. Review.
-
Ribose modified nucleosides and nucleotides as ligands for purine receptors.Nucleosides Nucleotides Nucleic Acids. 2001 Apr-Jul;20(4-7):333-41. doi: 10.1081/NCN-100002305. Nucleosides Nucleotides Nucleic Acids. 2001. PMID: 11563046 Free PMC article. Review.
Cited by
-
Resolution and concordance in dissecting the compound inhibitory junction potential.J Physiol. 2012 Apr 15;590(8):1777-8. doi: 10.1113/jphysiol.2012.230110. J Physiol. 2012. PMID: 22532644 Free PMC article. No abstract available.
-
Activation of P2Y1 nucleotide receptors induces inhibition of the M-type K+ current in rat hippocampal pyramidal neurons.J Neurosci. 2006 Sep 6;26(36):9340-8. doi: 10.1523/JNEUROSCI.2635-06.2006. J Neurosci. 2006. PMID: 16957090 Free PMC article.
-
2-Chloro N(6)-methyl-(N)-methanocarba-2'-deoxyadenosine-3',5'-bisphosphate is a selective high affinity P2Y(1) receptor antagonist.Br J Pharmacol. 2002 Apr;135(8):2004-10. doi: 10.1038/sj.bjp.0704673. Br J Pharmacol. 2002. PMID: 11959804 Free PMC article.
-
Adenine nucleotide analogues locked in a Northern methanocarba conformation: enhanced stability and potency as P2Y(1) receptor agonists.J Med Chem. 2002 May 9;45(10):2090-100. doi: 10.1021/jm010538v. J Med Chem. 2002. PMID: 11985476 Free PMC article.
-
β-Nicotinamide adenine dinucleotide acts at prejunctional adenosine A1 receptors to suppress inhibitory musculomotor neurotransmission in guinea pig colon and human jejunum.Am J Physiol Gastrointest Liver Physiol. 2015 Jun 1;308(11):G955-63. doi: 10.1152/ajpgi.00430.2014. Epub 2015 Mar 26. Am J Physiol Gastrointest Liver Physiol. 2015. PMID: 25813057 Free PMC article.
References
-
- North RA; Barnard EA Nucleotide receptors. Curr. Opin. NeuroBiol 1997, 7, 346–357. - PubMed
-
- Janssens R; Communi D; Pirotton S; Samson M; Parmentier M; Boeynaems JM Cloning and tissue distribution of the human P2Y1 receptor. Biochem. Biophys. Res. Commun 1996, 221, 588–593. - PubMed
-
- Webb TE; Simon J; Krishek BJ; Bateson AN; Smart TG; King BF; Burnstock G; Barnard EA Cloning and functional expression of a brain G-protein-coupled ATP receptor. FEBS Lett. 1993, 324, 219–225. - PubMed
-
- Jacobson KA; Kim Y-C; Camaioni E; van Rhee AM Structure activity relationships of P2 receptor agonists and antagonists. The P2 Nucleotide Receptors, in the series “The Receptors”; Humana Press: Clifton, NJ, 1997; pp 81–107.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Molecular Biology Databases

