H-ras but not K-ras traffics to the plasma membrane through the exocytic pathway
- PMID: 10713171
- PMCID: PMC85443
- DOI: 10.1128/MCB.20.7.2475-2487.2000
H-ras but not K-ras traffics to the plasma membrane through the exocytic pathway
Abstract
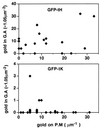
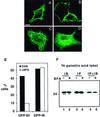
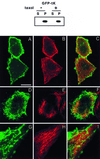
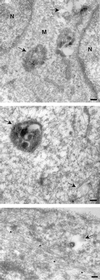
Ras proteins must be localized to the inner surface of the plasma membrane to be biologically active. The motifs that effect Ras plasma membrane targeting consist of a C-terminal CAAX motif plus a second signal comprising palmitoylation of adjacent cysteine residues or the presence of a polybasic domain. In this study, we examined how Ras proteins access the cell surface after processing of the CAAX motif is completed in the endoplasmic reticulum (ER). We show that palmitoylated CAAX proteins, in addition to being localized at the plasma membrane, are found throughout the exocytic pathway and accumulate in the Golgi region when cells are incubated at 15 degrees C. In contrast, polybasic CAAX proteins are found only at the cell surface and not in the exocytic pathway. CAAX proteins which lack a second signal for plasma membrane targeting accumulate in the ER and Golgi. Brefeldin A (BFA) significantly inhibits the plasma membrane accumulation of newly synthesized, palmitoylated CAAX proteins without inhibiting their palmitoylation. BFA has no effect on the trafficking of polybasic CAAX proteins. We conclude that H-ras and K-ras traffic to the cell surface through different routes and that the polybasic domain is a sorting signal diverting K-Ras out of the classical exocytic pathway proximal to the Golgi. Farnesylated Ras proteins that lack a polybasic domain reach the Golgi but require palmitoylation in order to traffic further to the cell surface. These data also indicate that a Ras palmitoyltransferase is present in an early compartment of the exocytic pathway.
Figures

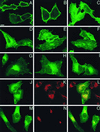
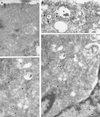
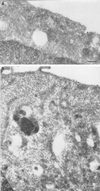
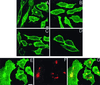
Similar articles
-
Palmitoylated Ras proteins traffic through recycling endosomes to the plasma membrane during exocytosis.J Cell Biol. 2010 Oct 4;191(1):23-9. doi: 10.1083/jcb.200911143. Epub 2010 Sep 27. J Cell Biol. 2010. PMID: 20876282 Free PMC article.
-
H-Ras dynamically interacts with recycling endosomes in CHO-K1 cells: involvement of Rab5 and Rab11 in the trafficking of H-Ras to this pericentriolar endocytic compartment.J Biol Chem. 2005 Oct 14;280(41):34997-5010. doi: 10.1074/jbc.M506256200. Epub 2005 Aug 3. J Biol Chem. 2005. PMID: 16079139
-
Endomembrane trafficking of ras: the CAAX motif targets proteins to the ER and Golgi.Cell. 1999 Jul 9;98(1):69-80. doi: 10.1016/S0092-8674(00)80607-8. Cell. 1999. PMID: 10412982
-
Ras proteins: different signals from different locations.Nat Rev Mol Cell Biol. 2003 May;4(5):373-84. doi: 10.1038/nrm1105. Nat Rev Mol Cell Biol. 2003. PMID: 12728271 Review.
-
Mechanisms of Ras protein targeting in mammalian cells.J Membr Biol. 2002 Nov 15;190(2):83-92. doi: 10.1007/s00232-002-1026-4. J Membr Biol. 2002. PMID: 12474073 Review.
Cited by
-
Optimized iLID Membrane Anchors for Local Optogenetic Protein Recruitment.ACS Synth Biol. 2021 May 21;10(5):1009-1023. doi: 10.1021/acssynbio.0c00511. Epub 2021 Apr 12. ACS Synth Biol. 2021. PMID: 33843200 Free PMC article.
-
In TCR-stimulated T-cells, N-ras regulates specific genes and signal transduction pathways.PLoS One. 2013 Jun 3;8(6):e63193. doi: 10.1371/journal.pone.0063193. Print 2014. PLoS One. 2013. PMID: 23755101 Free PMC article.
-
The Function of Embryonic Stem Cell-expressed RAS (E-RAS), a Unique RAS Family Member, Correlates with Its Additional Motifs and Its Structural Properties.J Biol Chem. 2015 Jun 19;290(25):15892-15903. doi: 10.1074/jbc.M115.640607. Epub 2015 May 4. J Biol Chem. 2015. PMID: 25940089 Free PMC article.
-
Haspin regulates Ras localization to promote Cdc24-driven mitotic depolarization.Cell Discov. 2020 Jun 23;6:42. doi: 10.1038/s41421-020-0170-2. eCollection 2020. Cell Discov. 2020. PMID: 32595981 Free PMC article.
-
Palmitoylated Ras proteins traffic through recycling endosomes to the plasma membrane during exocytosis.J Cell Biol. 2010 Oct 4;191(1):23-9. doi: 10.1083/jcb.200911143. Epub 2010 Sep 27. J Cell Biol. 2010. PMID: 20876282 Free PMC article.
References
-
- Aronheim A, Engelberg D, Li N, al Alawi N, Schlessinger J, Karin M. Membrane targeting of the nucleotide exchange factor Sos is sufficient for activating the Ras signaling pathway. Cell. 1994;78:949–961. - PubMed
-
- Booden M A, Baker T C, Solski P A, Der C J, Parker S G, Buss J E. A non-farnesylated Ha-Ras protein can be palmitylated and trigger potent differentiation and transformation. J Biol Chem. 1999;274:1423–1431. - PubMed
-
- Bos J L. ras oncogenes in human cancer: a review. Cancer Res. 1989;49:4682–4689. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
