Apg5p functions in the sequestration step in the cytoplasm-to-vacuole targeting and macroautophagy pathways
- PMID: 10712513
- PMCID: PMC14824
- DOI: 10.1091/mbc.11.3.969
Apg5p functions in the sequestration step in the cytoplasm-to-vacuole targeting and macroautophagy pathways
Abstract
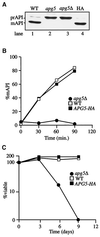
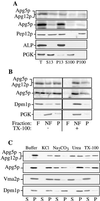
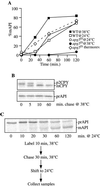
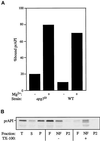
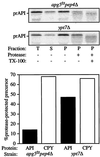
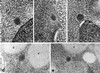
The cytoplasm-to-vacuole targeting (Cvt) pathway and macroautophagy are dynamic events involving the rearrangement of membrane to form a sequestering vesicle in the cytosol, which subsequently delivers its cargo to the vacuole. This process requires the concerted action of various proteins, including Apg5p. Recently, it was shown that another protein required for the import of aminopeptidase I (API) and autophagy, Apg12p, is covalently attached to Apg5p through the action of an E1-like enzyme, Apg7p. We have undertaken an analysis of Apg5p function to gain a better understanding of the role of this novel nonubiquitin conjugation reaction in these import pathways. We have generated the first temperature-sensitive mutant in the Cvt pathway, designated apg5(ts). Biochemical analysis of API import in the apg5(ts) strain confirmed that Apg5p is directly required for the import of API via the Cvt pathway. By analyzing the stage of API import that is blocked in the apg5(ts) mutant, we have determined that Apg5p is involved in the sequestration step and is required for vesicle formation and/or completion.
Figures



Similar articles
-
Apg7p/Cvt2p is required for the cytoplasm-to-vacuole targeting, macroautophagy, and peroxisome degradation pathways.Mol Biol Cell. 1999 May;10(5):1337-51. doi: 10.1091/mbc.10.5.1337. Mol Biol Cell. 1999. PMID: 10233148 Free PMC article.
-
Apg7p/Cvt2p: A novel protein-activating enzyme essential for autophagy.Mol Biol Cell. 1999 May;10(5):1367-79. doi: 10.1091/mbc.10.5.1367. Mol Biol Cell. 1999. PMID: 10233150 Free PMC article.
-
Apg16p is required for the function of the Apg12p-Apg5p conjugate in the yeast autophagy pathway.EMBO J. 1999 Jul 15;18(14):3888-96. doi: 10.1093/emboj/18.14.3888. EMBO J. 1999. PMID: 10406794 Free PMC article.
-
Autophagy, cytoplasm-to-vacuole targeting pathway, and pexophagy in yeast and mammalian cells.Annu Rev Biochem. 2000;69:303-42. doi: 10.1146/annurev.biochem.69.1.303. Annu Rev Biochem. 2000. PMID: 10966461 Review.
-
Transport of proteins to the yeast vacuole: autophagy, cytoplasm-to-vacuole targeting, and role of the vacuole in degradation.Semin Cell Dev Biol. 2000 Jun;11(3):173-9. doi: 10.1006/scdb.2000.0163. Semin Cell Dev Biol. 2000. PMID: 10906274 Review.
Cited by
-
The yeast Saccharomyces cerevisiae: an overview of methods to study autophagy progression.Methods. 2015 Mar;75:3-12. doi: 10.1016/j.ymeth.2014.12.008. Epub 2014 Dec 16. Methods. 2015. PMID: 25526918 Free PMC article. Review.
-
The Cytoplasm-to-Vacuole Targeting Pathway: A Historical Perspective.Int J Cell Biol. 2012;2012:142634. doi: 10.1155/2012/142634. Epub 2012 Feb 20. Int J Cell Biol. 2012. PMID: 22481942 Free PMC article.
-
Rab5 modulates aggregation and toxicity of mutant huntingtin through macroautophagy in cell and fly models of Huntington disease.J Cell Sci. 2008 May 15;121(Pt 10):1649-60. doi: 10.1242/jcs.025726. Epub 2008 Apr 22. J Cell Sci. 2008. PMID: 18430781 Free PMC article.
-
The development of MDA-7/IL-24 as a cancer therapeutic.Pharmacol Ther. 2010 Nov;128(2):375-84. doi: 10.1016/j.pharmthera.2010.08.001. Epub 2010 Aug 21. Pharmacol Ther. 2010. PMID: 20732354 Free PMC article. Review.
-
Kinetic assay of starvation sensitivity in yeast autophagy mutants allows for the identification of intermediary phenotypes.BMC Res Notes. 2019 Aug 14;12(1):505. doi: 10.1186/s13104-019-4545-0. BMC Res Notes. 2019. PMID: 31412956 Free PMC article.
References
-
- Hammond EM, Brunet CL, Johnson GD, Parkhill J, Milner AE, Brady G, Gregory CD, Grand RJ. Homology between a human apoptosis specific protein and the product of APG5, a gene involved in autophagy in yeast. FEBS Lett. 1998;425:391–395. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

