Hypoxia-inducible factor-1 mediates the biological effects of oxygen on human trophoblast differentiation through TGFbeta(3)
- PMID: 10712429
- PMCID: PMC289179
- DOI: 10.1172/JCI8316
Hypoxia-inducible factor-1 mediates the biological effects of oxygen on human trophoblast differentiation through TGFbeta(3)
Abstract
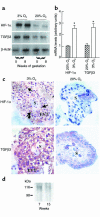
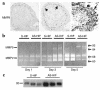
During early pregnancy, placentation occurs in a relatively hypoxic environment that is essential for appropriate embryonic development. Intervillous blood flow increases around 10 to 12 weeks of gestation and results in exposure of trophoblast cells to increased oxygen tension. Before this time, low oxygen appears to prevent trophoblast differentiation toward an invasive phenotype. Using human villous explants of 5-8 weeks' gestation, we found that low oxygen tension triggered trophoblast proliferation, fibronectin synthesis, alpha(5) integrin expression, and gelatinase A activity. These biochemical markers were barely detectable under oxic conditions. We therefore examined the placental expression of hypoxia-inducible factor-1 (HIF-1), a master regulator of oxygen homeostasis, and determined that expression of HIF-1alpha subunit during the first trimester of gestation parallels that of TGFbeta(3), an inhibitor of extravillous trophoblast differentiation. Expression of both molecules is high in early pregnancy and falls around 9 weeks of gestation, when placental pO(2) levels are believed to increase. Increasing oxygen tension induced a similar decrease in expression in cultured explants. Moreover, antisense inhibition of HIF-1alpha expression in hypoxic explants inhibited expression of TGFbeta(3), arrested cell proliferation, decreased alpha(5) expression and gelatinase A activity, and triggered biochemical markers of an invasive trophoblast phenotype such as alpha(1) integrin and gelatinase B expression. These data suggest that the oxygen-regulated early events of trophoblast differentiation are in part mediated by TGFbeta(3) through HIF-1 transcription factors.
Figures



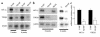


Comment in
-
Hypoxia and human placental development.J Clin Invest. 2000 Mar;105(5):559-60. doi: 10.1172/JCI9512. J Clin Invest. 2000. PMID: 10712424 Free PMC article. No abstract available.
Similar articles
-
Oxygen and placental development during the first trimester: implications for the pathophysiology of pre-eclampsia.Placenta. 2000 Mar-Apr;21 Suppl A:S25-30. doi: 10.1053/plac.1999.0522. Placenta. 2000. PMID: 10831118
-
Adriana and Luisa Castellucci Award lecture 2001. Hypoxia inducible factor-1: oxygen regulation of trophoblast differentiation in normal and pre-eclamptic pregnancies--a review.Placenta. 2002 Apr;23 Suppl A:S47-57. doi: 10.1053/plac.2002.0815. Placenta. 2002. PMID: 11978059 Review.
-
Hypoxia-inducible factor-1 transactivates transforming growth factor-beta3 in trophoblast.Endocrinology. 2004 Sep;145(9):4113-8. doi: 10.1210/en.2003-1639. Epub 2004 May 20. Endocrinology. 2004. PMID: 15155569
-
Hypoxia induced HIF-1/HIF-2 activity alters trophoblast transcriptional regulation and promotes invasion.Eur J Cell Biol. 2015 Dec;94(12):589-602. doi: 10.1016/j.ejcb.2015.10.004. Epub 2015 Oct 24. Eur J Cell Biol. 2015. PMID: 26531845
-
The regulation of trophoblast differentiation by oxygen in the first trimester of pregnancy.Hum Reprod Update. 2006 Mar-Apr;12(2):137-44. doi: 10.1093/humupd/dmi043. Epub 2005 Oct 18. Hum Reprod Update. 2006. PMID: 16234296 Review.
Cited by
-
Pre-Existing Diabetes Mellitus, Hypertension and KidneyDisease as Risk Factors of Pre-Eclampsia: A Disease of Theories and Its Association with Genetic Polymorphism.Int J Environ Res Public Health. 2022 Dec 12;19(24):16690. doi: 10.3390/ijerph192416690. Int J Environ Res Public Health. 2022. PMID: 36554576 Free PMC article. Review.
-
Sera from preeclampsia patients elicit symptoms of human disease in mice and provide a basis for an in vitro predictive assay.Am J Pathol. 2010 Nov;177(5):2387-98. doi: 10.2353/ajpath.2010.100475. Epub 2010 Oct 1. Am J Pathol. 2010. PMID: 20889559 Free PMC article.
-
Mesenchymal Stem/Stromal Cells Derived from Human and Animal Perinatal Tissues-Origins, Characteristics, Signaling Pathways, and Clinical Trials.Cells. 2021 Nov 23;10(12):3278. doi: 10.3390/cells10123278. Cells. 2021. PMID: 34943786 Free PMC article. Review.
-
Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche.Cancer Cell. 2009 Jan 6;15(1):35-44. doi: 10.1016/j.ccr.2008.11.012. Cancer Cell. 2009. PMID: 19111879 Free PMC article.
-
Placental Glucose Transporters and Response to Bisphenol A in Pregnancies from of Normal and Overweight Mothers.Int J Mol Sci. 2021 Jun 21;22(12):6625. doi: 10.3390/ijms22126625. Int J Mol Sci. 2021. PMID: 34205666 Free PMC article.
References
-
- Cross JC, Werb Z, Fisher SJ. Implantation and the placenta: key pieces of the development puzzle. Science. 1994;266:1508–1518. - PubMed
-
- Strickland S, Richards WG. Invasion of trophoblasts. Cell. 1992;71:355–357. - PubMed
-
- Aplin JD. Implantation, trophoblast differentiation and haemochorial placentation: mechanistic evidence in vivo and in vitro. J Cell Sci. 1991;99:681–692. - PubMed
-
- Rodesch F, Simon P, Donner C, Jauniaux E. Oxygen measurements in endometrial and trophoblastic tissues during early pregnancy. Obstet Gynecol. 1992;80:283–285. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

