Vaccinia virus envelope H3L protein binds to cell surface heparan sulfate and is important for intracellular mature virion morphogenesis and virus infection in vitro and in vivo
- PMID: 10708453
- PMCID: PMC111837
- DOI: 10.1128/jvi.74.7.3353-3365.2000
Vaccinia virus envelope H3L protein binds to cell surface heparan sulfate and is important for intracellular mature virion morphogenesis and virus infection in vitro and in vivo
Abstract
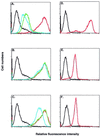
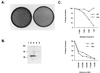
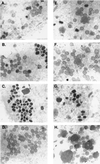
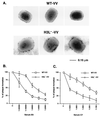
An immunodominant antigen, p35, is expressed on the envelope of intracellular mature virions (IMV) of vaccinia virus. p35 is encoded by the viral late gene H3L, but its role in the virus life cycle is not known. This report demonstrates that soluble H3L protein binds to heparan sulfate on the cell surface and competes with the binding of vaccinia virus, indicating a role for H3L protein in IMV adsorption to mammalian cells. A mutant virus defective in expression of H3L (H3L(-)) was constructed; the mutant virus has a small plaque phenotype and 10-fold lower IMV and extracellular enveloped virion titers than the wild-type virus. Virion morphogenesis is severely blocked and intermediate viral structures such as viral factories and crescents accumulate in cells infected with the H3L(-) mutant virus. IMV from the H3L(-) mutant virus are somewhat altered and less infectious than wild-type virions. However, cells infected by the mutant virus form multinucleated syncytia after low pH treatment, suggesting that H3L protein is not required for cell fusion. Mice inoculated intranasally with wild-type virus show high mortality and severe weight loss, whereas mice infected with H3L(-) mutant virus survive and recover faster, indicating that inactivation of the H3L gene attenuates virus virulence in vivo. In summary, these data indicate that H3L protein mediates vaccinia virus adsorption to cell surface heparan sulfate and is important for vaccinia virus infection in vitro and in vivo. In addition, H3L protein plays a role in virion assembly.
Figures





Similar articles
-
Effects of deletion or stringent repression of the H3L envelope gene on vaccinia virus replication.J Virol. 2000 Aug;74(16):7518-28. doi: 10.1128/jvi.74.16.7518-7528.2000. J Virol. 2000. PMID: 10906205 Free PMC article.
-
Vaccinia virus envelope D8L protein binds to cell surface chondroitin sulfate and mediates the adsorption of intracellular mature virions to cells.J Virol. 1999 Oct;73(10):8750-61. doi: 10.1128/JVI.73.10.8750-8761.1999. J Virol. 1999. PMID: 10482629 Free PMC article.
-
Vaccinia virus gene A36R encodes a M(r) 43-50 K protein on the surface of extracellular enveloped virus.Virology. 1994 Oct;204(1):376-90. doi: 10.1006/viro.1994.1542. Virology. 1994. PMID: 8091668
-
The formation and function of extracellular enveloped vaccinia virus.J Gen Virol. 2002 Dec;83(Pt 12):2915-2931. doi: 10.1099/0022-1317-83-12-2915. J Gen Virol. 2002. PMID: 12466468 Review.
-
The exit of vaccinia virus from infected cells.Virus Res. 2004 Dec;106(2):189-97. doi: 10.1016/j.virusres.2004.08.015. Virus Res. 2004. PMID: 15567497 Review.
Cited by
-
Monkeypox: epidemiology, pathogenesis, treatment and prevention.Signal Transduct Target Ther. 2022 Nov 2;7(1):373. doi: 10.1038/s41392-022-01215-4. Signal Transduct Target Ther. 2022. PMID: 36319633 Free PMC article. Review.
-
Identification of Diosmin and Flavin Adenine Dinucleotide as Repurposing Treatments for Monkeypox Virus: A Computational Study.Int J Mol Sci. 2022 Sep 30;23(19):11570. doi: 10.3390/ijms231911570. Int J Mol Sci. 2022. PMID: 36232872 Free PMC article.
-
Structural and functional analyses of viral H2 protein of the vaccinia virus entry fusion complex.J Virol. 2023 Dec 21;97(12):e0134323. doi: 10.1128/jvi.01343-23. Epub 2023 Nov 17. J Virol. 2023. PMID: 37975688 Free PMC article.
-
Molecular phylogenetics of a recently isolated goat pox virus from Vietnam.BMC Vet Res. 2021 Mar 8;17(1):115. doi: 10.1186/s12917-021-02777-1. BMC Vet Res. 2021. PMID: 33685458 Free PMC article.
-
Myxoma Virus Combination Therapy Enhances Lenalidomide and Bortezomib Treatments for Multiple Myeloma.Pathogens. 2024 Jan 12;13(1):72. doi: 10.3390/pathogens13010072. Pathogens. 2024. PMID: 38251379 Free PMC article.
References
-
- Appleyard G, Hapel A J, Boulter E A. An antigenic difference between intracellular and extracellular rabbitpox virus. J Gen Virol. 1971;13:9–17. - PubMed
-
- Banfield B W, Leduc Y, Esford L, Visalli R J, Brandt C R, Tufaro F. Evidence for an interaction of herpes simplex virus with chondroitin sulfate proteoglycans during infection. Virology. 1995;208:531–539. - PubMed
-
- Cardin A D, Weintraub H J R. Molecular modeling of protein-glycosaminoglycan interactions. Arteriosclerosis. 1989;9:21–32. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

