Fcgamma receptor-mediated phagocytosis in macrophages lacking the Src family tyrosine kinases Hck, Fgr, and Lyn
- PMID: 10684859
- PMCID: PMC2195832
- DOI: 10.1084/jem.191.4.669
Fcgamma receptor-mediated phagocytosis in macrophages lacking the Src family tyrosine kinases Hck, Fgr, and Lyn
Abstract
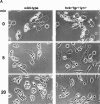
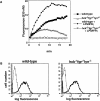
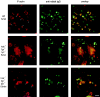
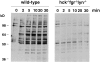
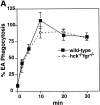
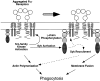
Macrophage Fcgamma receptors (FcgammaRs) mediate the uptake and destruction of antibody-coated viruses, bacteria, and parasites. We examined FcgammaR signaling and phagocytic function in bone marrow-derived macrophages from mutant mice lacking the major Src family kinases expressed in these cells, Hck, Fgr, and Lyn. Many FcgammaR-induced functional responses and signaling events were diminished or delayed in these macrophages, including immunoglobulin (Ig)G-coated erythrocyte phagocytosis, respiratory burst, actin cup formation, and activation of Syk, phosphatidylinositol 3-kinase, and extracellular signal-regulated kinases 1 and 2. Significant reduction of IgG-dependent phagocytosis was not seen in hck(-)(/)-fgr(-)(/)- or lyn(-)(/)- cells, although the single mutant lyn(-)(/)- macrophages did manifest signaling defects. Thus, Src family kinases clearly have roles in two events leading to FcgammaR-mediated phagocytosis, one involving initiation of actin polymerization and the second involving activation of Syk and subsequent internalization. Since FcgammaR-mediated phagocytosis did occur at modest levels in a delayed fashion in triple mutant macrophages, these Src family kinases are not absolutely required for uptake of IgG-opsonized particles.
Figures







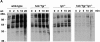
Similar articles
-
Role of Src kinases and Syk in Fcgamma receptor-mediated phagocytosis and phagosome-lysosome fusion.J Leukoc Biol. 2001 Nov;70(5):801-11. J Leukoc Biol. 2001. PMID: 11698501
-
A critical role for Syk in signal transduction and phagocytosis mediated by Fcgamma receptors on macrophages.J Exp Med. 1997 Oct 6;186(7):1027-39. doi: 10.1084/jem.186.7.1027. J Exp Med. 1997. PMID: 9314552 Free PMC article.
-
Differential involvement of Src family kinases in Fc gamma receptor-mediated phagocytosis.J Immunol. 2000 Jul 1;165(1):473-82. doi: 10.4049/jimmunol.165.1.473. J Immunol. 2000. PMID: 10861086
-
Lipopolysaccharide (LPS)-induced macrophage activation and signal transduction in the absence of Src-family kinases Hck, Fgr, and Lyn.J Exp Med. 1997 May 5;185(9):1661-70. doi: 10.1084/jem.185.9.1661. J Exp Med. 1997. PMID: 9151903 Free PMC article.
-
The p60c-src family of protein-tyrosine kinases: structure, regulation, and function.Crit Rev Oncog. 1992;3(4):401-46. Crit Rev Oncog. 1992. PMID: 1384720 Review.
Cited by
-
Current Understanding of Immune Thrombocytopenia: A Review of Pathogenesis and Treatment Options.Int J Mol Sci. 2024 Feb 10;25(4):2163. doi: 10.3390/ijms25042163. Int J Mol Sci. 2024. PMID: 38396839 Free PMC article. Review.
-
Structurally distinct phosphatases CD45 and CD148 both regulate B cell and macrophage immunoreceptor signaling.Immunity. 2008 Feb;28(2):183-96. doi: 10.1016/j.immuni.2007.11.024. Epub 2008 Jan 31. Immunity. 2008. PMID: 18249142 Free PMC article.
-
Hepatic Transcriptome Analysis Identifies Divergent Pathogen-Specific Targeting-Strategies to Modulate the Innate Immune System in Response to Intramammary Infection.Front Immunol. 2020 Apr 29;11:715. doi: 10.3389/fimmu.2020.00715. eCollection 2020. Front Immunol. 2020. PMID: 32411137 Free PMC article.
-
Endocytosis and the internalization of pathogenic organisms: focus on phosphoinositides.F1000Res. 2020 May 15;9:F1000 Faculty Rev-368. doi: 10.12688/f1000research.22393.1. eCollection 2020. F1000Res. 2020. PMID: 32494357 Free PMC article. Review.
-
Engineered proteins with sensing and activating modules for automated reprogramming of cellular functions.Nat Commun. 2017 Sep 7;8(1):477. doi: 10.1038/s41467-017-00569-6. Nat Commun. 2017. PMID: 28883531 Free PMC article.
References
-
- Daeron M. Fc receptor biology. Annu. Rev. Immunol. 1997;15:203–234. - PubMed
-
- Aderem A., Underhill D.M. Mechanisms of phagocytosis in macrophages. Annu. Rev. Immunol. 1999;17:593–623. - PubMed
-
- Deo Y.M., Graziano R.F., Repp R., van de Winkel J.G. Clinical significance of IgG Fc receptors and FcγR-directed immunotherapies. Immunol. Today. 1997;18:127–135. - PubMed
-
- Strzelecka A., Kwiatkowska K., Sobota A. Tyrosine phosphorylation and Fcγ receptor-mediated phagocytosis. FEBS Lett. 1997;400:11–14. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

