The intact retroviral Env glycoprotein of human foamy virus is a trimer
- PMID: 10684305
- PMCID: PMC111779
- DOI: 10.1128/jvi.74.6.2885-2887.2000
The intact retroviral Env glycoprotein of human foamy virus is a trimer
Abstract
Electron microscopy of negatively stained human foamy virus particles provides direct evidence for the trimeric nature of intact Env surface glycoproteins. Three-dimensional image reconstruction reveals that the Env trimer is a tapering spike 14 nm in length. The spikes were often arranged in hexagonal rings which shared adjacent Env trimers.
Figures

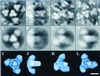
Similar articles
-
Cryo-electron Microscopy Structure of the Native Prototype Foamy Virus Glycoprotein and Virus Architecture.PLoS Pathog. 2016 Jul 11;12(7):e1005721. doi: 10.1371/journal.ppat.1005721. eCollection 2016 Jul. PLoS Pathog. 2016. PMID: 27399201 Free PMC article.
-
The roles of Pol and Env in the assembly pathway of human foamy virus.J Virol. 1998 May;72(5):3658-65. doi: 10.1128/JVI.72.5.3658-3665.1998. J Virol. 1998. PMID: 9557646 Free PMC article.
-
Sequential roles of receptor binding and low pH in forming prehairpin and hairpin conformations of a retroviral envelope glycoprotein.J Virol. 2004 Aug;78(15):8201-9. doi: 10.1128/JVI.78.15.8201-8209.2004. J Virol. 2004. PMID: 15254191 Free PMC article.
-
AIDS virus envelope spike structure.Curr Opin Struct Biol. 2007 Apr;17(2):244-52. doi: 10.1016/j.sbi.2007.03.008. Epub 2007 Mar 28. Curr Opin Struct Biol. 2007. PMID: 17395457 Review.
-
Insights into the trimeric HIV-1 envelope glycoprotein structure.Trends Biochem Sci. 2015 Feb;40(2):101-7. doi: 10.1016/j.tibs.2014.12.006. Epub 2015 Jan 16. Trends Biochem Sci. 2015. PMID: 25600289 Free PMC article. Review.
Cited by
-
The crystal structure of a simian Foamy Virus receptor binding domain provides clues about entry into host cells.Nat Commun. 2023 Mar 6;14(1):1262. doi: 10.1038/s41467-023-36923-0. Nat Commun. 2023. PMID: 36878926 Free PMC article.
-
The Unique, the Known, and the Unknown of Spumaretrovirus Assembly.Viruses. 2021 Jan 13;13(1):105. doi: 10.3390/v13010105. Viruses. 2021. PMID: 33451128 Free PMC article. Review.
-
Identification of an Intermediate Step in Foamy Virus Fusion.Viruses. 2020 Dec 21;12(12):1472. doi: 10.3390/v12121472. Viruses. 2020. PMID: 33371254 Free PMC article.
-
An Immunodominant and Conserved B-Cell Epitope in the Envelope of Simian Foamy Virus Recognized by Humans Infected with Zoonotic Strains from Apes.J Virol. 2019 May 15;93(11):e00068-19. doi: 10.1128/JVI.00068-19. Print 2019 Jun 1. J Virol. 2019. PMID: 30894477 Free PMC article.
-
Cryo-electron Microscopy Structure of the Native Prototype Foamy Virus Glycoprotein and Virus Architecture.PLoS Pathog. 2016 Jul 11;12(7):e1005721. doi: 10.1371/journal.ppat.1005721. eCollection 2016 Jul. PLoS Pathog. 2016. PMID: 27399201 Free PMC article.
References
-
- Fass D, Kim P S. Dissection of a retrovirus envelope protein reveals structural similarity to influenza hemagglutinin. Curr Biol. 1995;5:1377–1383. - PubMed
-
- Gelderblom H, Frank H. Spumavirinae. In: Nermut M V, Steven A C, editors. Animal virus structure. Amsterdam, The Netherlands: Elsevier Science Publishers; 1987. pp. 305–311.
-
- Grief C, Hockley D J, Fromholc C E, Kitchin P A. The morphology of simian immunodeficiency virus as shown by negative staining electron microscopy. J Gen Virol. 1999;70:2215–2219. - PubMed
-
- Hunter E. Viral entry and receptors. In: Coffin J M, Hughes S, Varmus H E, editors. Retroviruses. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1997. pp. 71–120. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

