Gelsolin deficiency blocks podosome assembly and produces increased bone mass and strength
- PMID: 10684249
- PMCID: PMC2169374
- DOI: 10.1083/jcb.148.4.665
Gelsolin deficiency blocks podosome assembly and produces increased bone mass and strength
Abstract
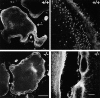
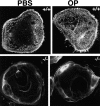
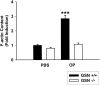
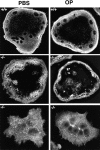
Osteoclasts are unique cells that utilize podosomes instead of focal adhesions for matrix attachment and cytoskeletal remodeling during motility. We have shown that osteopontin (OP) binding to the alpha(v)beta(3) integrin of osteoclast podosomes stimulated cytoskeletal reorganization and bone resorption by activating a heteromultimeric signaling complex that includes gelsolin, pp(60c-src), and phosphatidylinositol 3'-kinase. Here we demonstrate that gelsolin deficiency blocks podosome assembly and alpha(v)beta(3)-stimulated signaling related to motility in gelsolin-null mice. Gelsolin-deficient osteoclasts were hypomotile due to retarded remodeling of the actin cytoskeleton. They failed to respond to the autocrine factor, OP, with stimulation of motility and bone resorption. Gelsolin deficiency was associated with normal skeletal development and endochondral bone growth. However, gelsolin-null mice had mildly abnormal epiphyseal structure, retained cartilage proteoglycans in metaphyseal trabeculae, and increased trabecular thickness. With age, the gelsolin-deficient mice expressed increased trabecular and cortical bone thickness producing mechanically stronger bones. These observations demonstrate the critical role of gelsolin in podosome assembly, rapid cell movements, and signal transduction through the alpha(v)beta(3) integrin.
Figures


Similar articles
-
Polyphosphoinositides-dependent regulation of the osteoclast actin cytoskeleton and bone resorption.BMC Cell Biol. 2004 May 13;5:19. doi: 10.1186/1471-2121-5-19. BMC Cell Biol. 2004. PMID: 15142256 Free PMC article.
-
Rho-A is critical for osteoclast podosome organization, motility, and bone resorption.J Biol Chem. 2000 Apr 21;275(16):11993-2002. doi: 10.1074/jbc.275.16.11993. J Biol Chem. 2000. PMID: 10766830
-
c-Src is required for stimulation of gelsolin-associated phosphatidylinositol 3-kinase.J Biol Chem. 1998 May 8;273(19):11908-16. doi: 10.1074/jbc.273.19.11908. J Biol Chem. 1998. PMID: 9565618
-
Regulation of podosomes by integrin alphavbeta3 and Rho GTPase-facilitated phosphoinositide signaling.Eur J Cell Biol. 2006 Apr;85(3-4):311-7. doi: 10.1016/j.ejcb.2006.01.008. Epub 2006 Feb 7. Eur J Cell Biol. 2006. PMID: 16460838 Review.
-
Integrin-associated molecules and signalling cross talking in osteoclast cytoskeleton regulation.J Cell Mol Med. 2020 Mar;24(6):3271-3281. doi: 10.1111/jcmm.15052. Epub 2020 Feb 11. J Cell Mol Med. 2020. PMID: 32045092 Free PMC article. Review.
Cited by
-
Proteomic analysis of circulating monocytes in Chinese premenopausal females with extremely discordant bone mineral density.Proteomics. 2008 Oct;8(20):4259-72. doi: 10.1002/pmic.200700480. Proteomics. 2008. PMID: 18924182 Free PMC article.
-
Deciphering the involvement of the Hippo pathway co-regulators, YAP/TAZ in invadopodia formation and matrix degradation.Cell Death Dis. 2023 Apr 25;14(4):290. doi: 10.1038/s41419-023-05769-1. Cell Death Dis. 2023. PMID: 37185904 Free PMC article.
-
Osteopontin deficiency produces osteoclast dysfunction due to reduced CD44 surface expression.Mol Biol Cell. 2003 Jan;14(1):173-89. doi: 10.1091/mbc.e02-06-0354. Mol Biol Cell. 2003. PMID: 12529435 Free PMC article.
-
Plasma Gelsolin: Indicator of Inflammation and Its Potential as a Diagnostic Tool and Therapeutic Target.Int J Mol Sci. 2018 Aug 25;19(9):2516. doi: 10.3390/ijms19092516. Int J Mol Sci. 2018. PMID: 30149613 Free PMC article. Review.
-
Is GSN significant for hip BMD in female Caucasians?Bone. 2014 Jun;63:69-75. doi: 10.1016/j.bone.2014.02.015. Epub 2014 Mar 4. Bone. 2014. PMID: 24607942 Free PMC article.
References
-
- Arora P.D., McCulloch C.A.G. Dependence of fibroblast migration on actin severing activity of gelsolin. J. Biol. Chem. 1996;271:20516–20523. - PubMed
-
- Barkalow K., Hartwig J.H. Actin cytoskeleton. Setting the pace of cell movement. Curr. Biol. 1995;5:1000–1002. - PubMed
-
- Blair H.C., Teitelbaum S.L., Ghiselli R., Gluck S. Osteoclastic bone resorption by a polarized vacuolar proton pump. Nature. 1989;245:855–857. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

