Engineered herpes simplex virus expressing IL-12 in the treatment of experimental murine brain tumors
- PMID: 10681459
- PMCID: PMC15779
- DOI: 10.1073/pnas.040557897
Engineered herpes simplex virus expressing IL-12 in the treatment of experimental murine brain tumors
Abstract
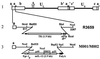
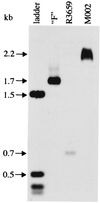
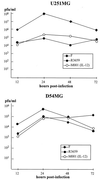
Genetically engineered, neuroattenuated herpes simplex viruses (HSVs) expressing various cytokines can improve survival when used in the treatment of experimental brain tumors. These attenuated viruses have both copies of gamma(1)34.5 deleted. Recently, we demonstrated increased survival of C57BL/6 mice bearing syngeneic GL-261 gliomas when treated with an engineered HSV expressing IL-4, as compared with treatment with the parent construct (gamma(1)34. 5(-)) alone or with a virus expressing IL-10. Herein, we report construction of a conditionally replication-competent mutant expressing both subunits of mIL-12 (M002) and its evaluation in a syngeneic neuroblastoma murine model. IL-12 induces a helper T cell subset type 1 response, which may induce more durable antitumor effects. In vitro studies showed that, when infected with M002, both Vero cells and murine Neuro-2a neuroblastoma cells produced physiologically relevant levels of IL-12 heterodimers, as determined by ELISA. M002 was cytotoxic for Neuro-2a cells and human glioma cell lines U251MG and D54MG. Neurotoxicity studies, as defined by plaque-forming units/LD(50), performed in HSV-1-sensitive A/J strain mice found that M002 was not toxic even at high doses. When evaluated in an intracranial syngeneic neuroblastoma murine model, median survival of M002-treated animals was significantly longer than the median survival of animals treated with R3659, the parent gamma(1)34.5(-) mutant lacking any cytokine gene insert. Immunohistochemical analysis of M002-treated tumors identified a pronounced influx of CD4(+) T cells and macrophages as well as CD8(+) cells when compared with an analysis of R3659-treated tumors. We conclude that M002 produced a survival benefit via oncolytic effects combined with immunologic effects meditated by helper T cells of subset type 1.
Figures


Similar articles
-
Serial passage through human glioma xenografts selects for a Deltagamma134.5 herpes simplex virus type 1 mutant that exhibits decreased neurotoxicity and prolongs survival of mice with experimental brain tumors.J Virol. 2006 Aug;80(15):7308-15. doi: 10.1128/JVI.00725-06. J Virol. 2006. PMID: 16840311 Free PMC article.
-
Preclinical evaluation of a genetically engineered herpes simplex virus expressing interleukin-12.J Virol. 2012 May;86(9):5304-13. doi: 10.1128/JVI.06998-11. Epub 2012 Feb 29. J Virol. 2012. PMID: 22379082 Free PMC article.
-
Enhanced inhibition of syngeneic murine tumors by combinatorial therapy with genetically engineered HSV-1 expressing CCL2 and IL-12.Cancer Gene Ther. 2005 Apr;12(4):359-68. doi: 10.1038/sj.cgt.7700784. Cancer Gene Ther. 2005. PMID: 15678154
-
The application of genetically engineered herpes simplex viruses to the treatment of experimental brain tumors.Proc Natl Acad Sci U S A. 1996 Oct 15;93(21):11313-8. doi: 10.1073/pnas.93.21.11313. Proc Natl Acad Sci U S A. 1996. PMID: 8876132 Free PMC article. Review.
-
Oncolytic virus therapy using genetically engineered herpes simplex viruses.Hum Cell. 2002 Sep;15(3):151-9. doi: 10.1111/j.1749-0774.2002.tb00109.x. Hum Cell. 2002. PMID: 12703545 Review.
Cited by
-
Viral therapy of glioblastoma multiforme.Proc Natl Acad Sci U S A. 2013 Jul 16;110(29):11672-3. doi: 10.1073/pnas.1310253110. Epub 2013 Jul 8. Proc Natl Acad Sci U S A. 2013. PMID: 23836634 Free PMC article. No abstract available.
-
Advances in Anti-Cancer Immunotherapy: Car-T Cell, Checkpoint Inhibitors, Dendritic Cell Vaccines, and Oncolytic Viruses, and Emerging Cellular and Molecular Targets.Cancers (Basel). 2020 Jul 7;12(7):1826. doi: 10.3390/cancers12071826. Cancers (Basel). 2020. PMID: 32645977 Free PMC article. Review.
-
Oncolytic virus therapy for glioblastoma multiforme: concepts and candidates.Cancer J. 2012 Jan-Feb;18(1):69-81. doi: 10.1097/PPO.0b013e31824671c9. Cancer J. 2012. PMID: 22290260 Free PMC article. Review.
-
Immunotherapy for glioblastoma: current state, challenges, and future perspectives.Cell Mol Immunol. 2024 Dec;21(12):1354-1375. doi: 10.1038/s41423-024-01226-x. Epub 2024 Oct 15. Cell Mol Immunol. 2024. PMID: 39406966 Free PMC article. Review.
-
The emerging field of viroimmunotherapy for pediatric brain tumors.Neuro Oncol. 2024 Nov 4;26(11):1981-1993. doi: 10.1093/neuonc/noae160. Neuro Oncol. 2024. PMID: 39148489 Review.
References
-
- Markert, J. M., Gillespie, G. Y., Weichselbaum, R. R., Roizman, B. & Whitley, R. J. (1999) Rev. Med. Virol., in press. - PubMed
-
- Cobbs C, Markert J. Persp Neurolog Surg. 1999;10:1–20.
-
- Martuza R L, Malick A, Markert J M, Ruffner K L, Coen D M. Science. 1991;252:854–856. - PubMed
-
- Mineta T, Rabkin S D, Yazaki T, Hunter W D, Martuza R L. Nat Med. 1995;1:938–943. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

