Consequences of placing an intramolecular crosslink in myosin S1
- PMID: 10677484
- PMCID: PMC26456
- DOI: 10.1073/pnas.030523997
Consequences of placing an intramolecular crosslink in myosin S1
Abstract
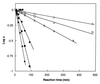
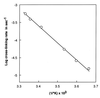
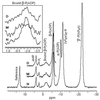
This paper describes the placement of a crosslinking agent (dibromobimane) between two thiols (Cys-522 and Cys-707) of a fragment, "S1," of the motor protein, myosin. It turns out that fastening the first anchor of the crosslinker is easy and rapid, but fastening the second anchor (Cys-522) is very temperature dependent, taking 30 min at room temperature but about a week on ice. Moreover, crystallography taken at 4 degrees C would seem to predict that the linkage is impossible, because the span of the crosslinking agent is much less than the interthiol distance. The simplest resolution of this seeming paradox is that structural fluctuations of the protein render the linkage increasingly likely as the temperature increases. Also, measurements of the affinity of MgADP for the protein, as well as the magnetic resonance of the P-atoms of the ADP once emplaced, suggest that binding the first reagent anchor to Cys-707 initiates an influence that travels to the rather distant ADP-binding site, and it is speculated what this "path of influence" might be.
Figures

Similar articles
-
Thiol-specific cross-linkers of variable length reveal a similar separation of SH1 and SH2 in myosin subfragment 1 in the presence and absence of MgADP.Biochemistry. 1999 Aug 10;38(32):10307-17. doi: 10.1021/bi990615c. Biochemistry. 1999. PMID: 10441124
-
Is SH1-SH2-cross-linked myosin subfragment 1 a structural analog of the weakly-bound state of myosin?Biophys J. 2000 Jul;79(1):460-7. doi: 10.1016/S0006-3495(00)76307-7. Biophys J. 2000. PMID: 10866971 Free PMC article.
-
Probing the conformational states of the SH1-SH2 helix in myosin: a cross-linking approach.Biochemistry. 1998 Nov 24;37(47):16704-10. doi: 10.1021/bi9817212. Biochemistry. 1998. PMID: 9843439
-
Temperature-induced changes in the flexibility of the loop between SH1 (Cys-707) and SH3 (Cys-522) in myosin subfragment 1 detected by cross-linking.Arch Biochem Biophys. 1991 Oct;290(1):1-6. doi: 10.1016/0003-9861(91)90583-5. Arch Biochem Biophys. 1991. PMID: 1898079
-
[Calorimetric study of stable complexes of myosin subfragment I with adenosine diphosphate and phosphate analogs].Biofizika. 1996 Jan-Feb;41(1):64-72. Biofizika. 1996. PMID: 8714460 Review. Russian.
Cited by
-
Myosin as a potential redox-sensor: an in vitro study.J Muscle Res Cell Motil. 2008;29(2-5):119-26. doi: 10.1007/s10974-008-9145-x. Epub 2008 Sep 9. J Muscle Res Cell Motil. 2008. PMID: 18780150
-
On the tryptophan residue of smooth muscle myosin that responds to binding of nucleotide.Proc Natl Acad Sci U S A. 2000 Oct 10;97(21):11203-8. doi: 10.1073/pnas.200362897. Proc Natl Acad Sci U S A. 2000. PMID: 11016961 Free PMC article.
-
Effects of oxidative stress on behavior, physiology, and the redox thiol proteome of Caenorhabditis elegans.Antioxid Redox Signal. 2011 Mar 15;14(6):1023-37. doi: 10.1089/ars.2010.3203. Epub 2010 Oct 28. Antioxid Redox Signal. 2011. PMID: 20649472 Free PMC article.
-
Quantitative evaluation of the lengths of homobifunctional protein cross-linking reagents used as molecular rulers.Protein Sci. 2001 Jul;10(7):1293-304. doi: 10.1110/ps.51201. Protein Sci. 2001. PMID: 11420431 Free PMC article.
References
-
- Ue K. Biochemistry. 1987;26:1889–1804. - PubMed
-
- Kosower E M, Kosower N S. Methods Enzymol. 1995;12:133–166. - PubMed
-
- Agarwal R, Burke M. Arch Biochem Biophys. 1991;290:1–6. - PubMed
-
- Rayment I, Rypnewski W R, Schmidt-Base K, Smith R, Tomchik D R, Benning M, Winkelmann D, Wesenberg G, Holden H M. Science. 1993;261:50–59. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

