Hsp15: a ribosome-associated heat shock protein
- PMID: 10675343
- PMCID: PMC305612
- DOI: 10.1093/emboj/19.4.741
Hsp15: a ribosome-associated heat shock protein
Abstract
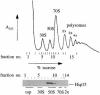
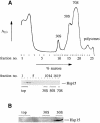
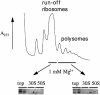
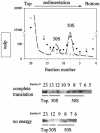
We are analyzing highly conserved heat shock genes of unknown or unclear function with the aim of determining their cellular role. Hsp15 has previously been shown to be an abundant nucleic acid-binding protein whose synthesis is induced massively at the RNA level upon temperature upshift. We have now identified that the in vivo target of Hsp15 action is the free 50S ribosomal subunit. Hsp15 binds with very high affinity (K(D) <5 nM) to this subunit, but only when 50S is free, not when it is part of the 70S ribosome. In addition, the binding of Hsp15 appears to correlate with a specific state of the mature, free 50S subunit, which contains bound nascent chain. This provides the first evidence for a so far unrecognized abortive event in translation. Hsp15 is suggested to be involved in the recycling of free 50S subunits that still carry a nascent chain. This gives Hsp15 a very different functional role from all other heat shock proteins and points to a new aspect of translation.
Figures



Similar articles
-
Recycling of aborted ribosomal 50S subunit-nascent chain-tRNA complexes by the heat shock protein Hsp15.J Mol Biol. 2009 Mar 13;386(5):1357-67. doi: 10.1016/j.jmb.2008.10.079. Epub 2008 Nov 5. J Mol Biol. 2009. PMID: 19013177
-
Structure of Escherichia coli heat shock protein Hsp15 in complex with the ribosomal 50S subunit bearing peptidyl-tRNA.Nucleic Acids Res. 2022 Nov 28;50(21):12515-12526. doi: 10.1093/nar/gkac1035. Nucleic Acids Res. 2022. PMID: 36370110 Free PMC article.
-
Novel heat shock protein HspQ stimulates the degradation of mutant DnaA protein in Escherichia coli.Genes Cells. 2004 Dec;9(12):1151-66. doi: 10.1111/j.1365-2443.2004.00800.x. Genes Cells. 2004. PMID: 15569148
-
Folding of a nascent peptide on the ribosome.Prog Nucleic Acid Res Mol Biol. 2001;66:41-66. doi: 10.1016/s0079-6603(00)66026-9. Prog Nucleic Acid Res Mol Biol. 2001. PMID: 11051761 Review.
-
Cold shock and adaptation.Bioessays. 1998 Jan;20(1):49-57. doi: 10.1002/(SICI)1521-1878(199801)20:1<49::AID-BIES8>3.0.CO;2-N. Bioessays. 1998. PMID: 9504047 Review.
Cited by
-
Co-expression of Skp and FkpA chaperones improves cell viability and alters the global expression of stress response genes during scFvD1.3 production.Microb Cell Fact. 2010 Apr 13;9:22. doi: 10.1186/1475-2859-9-22. Microb Cell Fact. 2010. PMID: 20388215 Free PMC article.
-
Computational genome-wide identification of heat shock protein genes in the bovine genome.F1000Res. 2018 Sep 20;7:1504. doi: 10.12688/f1000research.16058.1. eCollection 2018. F1000Res. 2018. PMID: 30542619 Free PMC article.
-
Small heat shock proteins from extremophiles: a review.Extremophiles. 2004 Feb;8(1):1-11. doi: 10.1007/s00792-003-0362-3. Epub 2003 Nov 19. Extremophiles. 2004. PMID: 15064984 Review.
-
YbeY, a heat shock protein involved in translation in Escherichia coli.J Bacteriol. 2009 Apr;191(8):2649-55. doi: 10.1128/JB.01663-08. Epub 2009 Jan 30. J Bacteriol. 2009. PMID: 19181801 Free PMC article.
-
Ribosome Rescue Pathways in Bacteria.Front Microbiol. 2021 Mar 18;12:652980. doi: 10.3389/fmicb.2021.652980. eCollection 2021. Front Microbiol. 2021. PMID: 33815344 Free PMC article. Review.
References
-
- Aravind L. and Koonin, E.V. (1999) Novel predicted RNA-binding domains associated with the translation machinery. J. Mol. Evol., 48, 291–302. - PubMed
-
- Becker J. and Craig, E.A. (1994) Heat-shock proteins as molecular chaperones. Eur. J. Biochem., 219, 11–23. - PubMed
-
- Blattner F.R., et al. (1997)The complete genome sequence of Escherichia coli K-12. Science, 277, 1453–1474. - PubMed
-
- Bommer U.A., Burkhardt,N., Jünemann,R., Spahn,C.M.T., Triana-Alonso,F.J. and Nierhaus,K.H. (1996) Ribosomes and polysomes. In Graham,J. and Rickwoods,D. (eds), Subcellular Fractionation. A Practical Approach. IRL Press, Oxford, UK, pp. 271–301.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

