A discrete subpopulation of dendritic cells transports apoptotic intestinal epithelial cells to T cell areas of mesenteric lymph nodes
- PMID: 10662789
- PMCID: PMC2195813
- DOI: 10.1084/jem.191.3.435
A discrete subpopulation of dendritic cells transports apoptotic intestinal epithelial cells to T cell areas of mesenteric lymph nodes
Abstract
This study identifies a dendritic cell (DC) subset that constitutively transports apoptotic intestinal epithelial cell remnants to T cell areas of mesenteric lymph nodes in vivo. Rat intestinal lymph contains two DC populations. Both populations have typical DC morphology, are major histocompatibility complex class II(hi), and express OX62, CD11c, and B7. CD4(+)/OX41(+) DCs are strong antigen-presenting cells (APCs). CD4(-)/OX41(-) DCs are weak APCs and contain cytoplasmic apoptotic DNA, epithelial cell-restricted cytokeratins, and nonspecific esterase (NSE)(+) inclusions, not seen in OX41(+) DCs. Identical patterns of NSE electrophoretic variants exist in CD4(-)/OX41(-) DCs, intestinal epithelial cells, and mesenteric node DCs but not in other DC populations, macrophages, or tissues. Terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick-end labeling (TUNEL)-positive DCs and strongly NSE(+) DCs are present in intestinal lamina propria. Peyer's patches and mesenteric but not other lymph nodes contain many strongly NSE(+) DCs in interfollicular and T cell areas. Similar DCs are seen in the ileum and in T cell areas of mesenteric nodes in gnotobiotic rats. These results show that a distinct DC subset constitutively endocytoses and transports apoptotic cells to T cell areas and suggest a role for these DCs in inducing and maintaining peripheral self-tolerance.
Figures
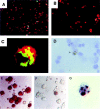
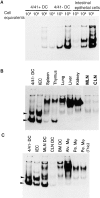
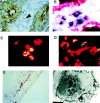


Comment in
-
The induction of tolerance by dendritic cells that have captured apoptotic cells.J Exp Med. 2000 Feb 7;191(3):411-6. doi: 10.1084/jem.191.3.411. J Exp Med. 2000. PMID: 10662786 Free PMC article. No abstract available.
Similar articles
-
Dendritic cell heterogeneity in vivo: two functionally different dendritic cell populations in rat intestinal lymph can be distinguished by CD4 expression.J Immunol. 1998 Aug 1;161(3):1146-55. J Immunol. 1998. PMID: 9686573
-
CCR7 is critically important for migration of dendritic cells in intestinal lamina propria to mesenteric lymph nodes.J Immunol. 2006 Jan 15;176(2):803-10. doi: 10.4049/jimmunol.176.2.803. J Immunol. 2006. PMID: 16393963
-
Intestinal dendritic cell subsets: differential effects of systemic TLR4 stimulation on migratory fate and activation in vivo.J Immunol. 2005 Feb 1;174(3):1374-84. doi: 10.4049/jimmunol.174.3.1374. J Immunol. 2005. PMID: 15661895
-
Phenotype and function of intestinal dendritic cells.Semin Immunol. 2005 Aug;17(4):284-94. doi: 10.1016/j.smim.2005.05.010. Semin Immunol. 2005. PMID: 15978836 Review.
-
Subsets of migrating intestinal dendritic cells.Immunol Rev. 2010 Mar;234(1):259-67. doi: 10.1111/j.0105-2896.2009.00866.x. Immunol Rev. 2010. PMID: 20193024 Review.
Cited by
-
Why do intestinal epithelial cells express MHC class II?Immunology. 2021 Apr;162(4):357-367. doi: 10.1111/imm.13270. Epub 2020 Oct 12. Immunology. 2021. PMID: 32966619 Free PMC article. Review.
-
Combining radiotherapy and immunotherapy: a revived partnership.Int J Radiat Oncol Biol Phys. 2005 Nov 1;63(3):655-66. doi: 10.1016/j.ijrobp.2005.06.032. Int J Radiat Oncol Biol Phys. 2005. PMID: 16199306 Free PMC article. Review.
-
In vitro atrazine exposure affects the phenotypic and functional maturation of dendritic cells.Toxicol Appl Pharmacol. 2007 Sep 15;223(3):206-17. doi: 10.1016/j.taap.2007.06.004. Epub 2007 Jun 21. Toxicol Appl Pharmacol. 2007. PMID: 17662328 Free PMC article.
-
Homeostasis and inflammation in the intestine.Cell. 2010 Mar 19;140(6):859-70. doi: 10.1016/j.cell.2010.01.023. Cell. 2010. PMID: 20303876 Free PMC article. Review.
-
In vivo maturation and migration of dendritic cells.Immunology. 2001 Mar;102(3):255-62. doi: 10.1046/j.1365-2567.2001.01204.x. Immunology. 2001. PMID: 11298823 Free PMC article. Review. No abstract available.
References
-
- Banchereau J., Steinman R.M. Dendritic cells and the control of immunity. Nature. 1998;392:245–252 . - PubMed
-
- Lanzavecchia A. Mechanisms of antigen uptake for presentation. Curr. Opin. Immunol. 1996;8:348–354. - PubMed
-
- Zocchi M.R., Poggi A., Rubartelli A. The RGD-containing domain of exogenous HIV-1 Tat inhibits the engulfment of apoptotic bodies by dendritic cells. AIDS. 1997;11:1227–1235. - PubMed
-
- Rovere P., Manfredi A.A., Vallinoto C., Zimmermann V.S., Fascio U., Balestrieri G., Ricciardi Castagnoli P., Rugarli C., Tincani A. Dendritic cells preferentially internalize apoptotic cells opsonized by anti-β2-glycoprotein I antibodies. J. Autoimmun. 1998;11:403–411. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

