Toward a protein-protein interaction map of the budding yeast: A comprehensive system to examine two-hybrid interactions in all possible combinations between the yeast proteins
- PMID: 10655498
- PMCID: PMC15550
- DOI: 10.1073/pnas.97.3.1143
Toward a protein-protein interaction map of the budding yeast: A comprehensive system to examine two-hybrid interactions in all possible combinations between the yeast proteins
Abstract
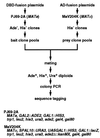
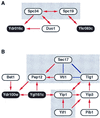
Protein-protein interactions play pivotal roles in various aspects of the structural and functional organization of the cell, and their complete description is indispensable to thorough understanding of the cell. As an approach toward this goal, here we report a comprehensive system to examine two-hybrid interactions in all of the possible combinations between proteins of Saccharomyces cerevisiae. We cloned all of the yeast ORFs individually as a DNA-binding domain fusion ("bait") in a MATa strain and as an activation domain fusion ("prey") in a MATalpha strain, and subsequently divided them into pools, each containing 96 clones. These bait and prey clone pools were systematically mated with each other, and the transformants were subjected to strict selection for the activation of three reporter genes followed by sequence tagging. Our initial examination of approximately 4 x 10(6) different combinations, constituting approximately 10% of the total to be tested, has revealed 183 independent two-hybrid interactions, more than half of which are entirely novel. Notably, the obtained binary data allow us to extract more complex interaction networks, including the one that may explain a currently unsolved mechanism for the connection between distinct steps of vesicular transport. The approach described here thus will provide many leads for integration of various cellular functions and serve as a major driving force in the completion of the protein-protein interaction map.
Figures
Similar articles
-
Yeast two-hybrid screening.Methods Mol Biol. 2007;362:207-23. doi: 10.1007/978-1-59745-257-1_15. Methods Mol Biol. 2007. PMID: 17417012
-
A comprehensive two-hybrid analysis to explore the yeast protein interactome.Proc Natl Acad Sci U S A. 2001 Apr 10;98(8):4569-74. doi: 10.1073/pnas.061034498. Epub 2001 Mar 13. Proc Natl Acad Sci U S A. 2001. PMID: 11283351 Free PMC article.
-
High-throughput yeast two-hybrid assays for large-scale protein interaction mapping.Methods. 2001 Jul;24(3):297-306. doi: 10.1006/meth.2001.1190. Methods. 2001. PMID: 11403578
-
Analysing protein-protein interactions with the yeast two-hybrid system.Plant Mol Biol. 2002 Dec;50(6):855-70. doi: 10.1023/a:1021214007897. Plant Mol Biol. 2002. PMID: 12516858 Review.
-
Results and prospects of the yeast three-hybrid system.FEBS Lett. 2004 Jan 2;556(1-3):7-12. doi: 10.1016/s0014-5793(03)01434-0. FEBS Lett. 2004. PMID: 14706817 Review.
Cited by
-
In Vivo Glutathione S-Transferases Superfamily Proteome Analysis: An Insight into Aedes albopictus Mosquitoes upon Acute Xenobiotic Challenges.Insects. 2022 Nov 7;13(11):1028. doi: 10.3390/insects13111028. Insects. 2022. PMID: 36354852 Free PMC article.
-
Direct selection on genetic robustness revealed in the yeast transcriptome.PLoS One. 2007 Sep 19;2(9):e911. doi: 10.1371/journal.pone.0000911. PLoS One. 2007. PMID: 17878946 Free PMC article.
-
RNase H2 of Saccharomyces cerevisiae is a complex of three proteins.Nucleic Acids Res. 2004 Jan 20;32(2):407-14. doi: 10.1093/nar/gkh209. Print 2004. Nucleic Acids Res. 2004. PMID: 14734815 Free PMC article.
-
A proteome-wide protein interaction map for Campylobacter jejuni.Genome Biol. 2007;8(7):R130. doi: 10.1186/gb-2007-8-7-r130. Genome Biol. 2007. PMID: 17615063 Free PMC article.
-
Interactions of rotavirus VP4 spike protein with the endosomal protein Rab5 and the prenylated Rab acceptor PRA1.J Virol. 2003 Jun;77(12):7041-7. doi: 10.1128/jvi.77.12.7041-7047.2003. J Virol. 2003. PMID: 12768023 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

