Metabotropic glutamate receptors trigger homosynaptic protein synthesis to prolong long-term potentiation
- PMID: 10648701
- PMCID: PMC6774154
- DOI: 10.1523/JNEUROSCI.20-03-00969.2000
Metabotropic glutamate receptors trigger homosynaptic protein synthesis to prolong long-term potentiation
Abstract
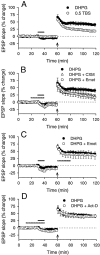
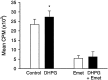
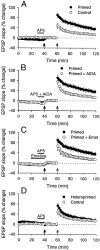
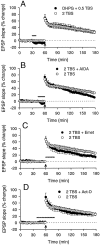
We investigated the mechanisms by which previous "priming" activation of group I metabotropic glutamate receptors (mGluRs) facilitates the persistence of long-term potentiation (LTP) in area CA1 of rat hippocampal slices. Priming of LTP was elicited by either pharmacological or synaptic activation of mGluRs before a weak tetanic stimulus that normally produced only a rapidly decaying phase of LTP that did not involve protein synthesis or mGluRs. Pharmacological priming of LTP persistence by a selective group I mGluR agonist was blocked by an inhibitor of group I mGluRs and by inhibitors of translation, but not by a transcriptional inhibitor. The same mGluR agonist increased (35)S-methionine incorporation into slice proteins. LTP could also be facilitated using a synaptic stimulation priming protocol, and this effect was similarly blocked by group I mGluR and protein synthesis inhibitors. Furthermore, using a two-pathway protocol, the synaptic priming of LTP was found to be input-specific. To test for the contribution of group I mGluRs and protein synthesis to LTP in nonprimed slices, a longer duration control tetanization protocol was used to elicit a more slowly decaying form of LTP than did the weak tetanus used in the previous experiments. The persistence of the LTP induced by this stronger tetanus was dependent on mGluR activation and protein synthesis but not on transcription. Together, these results suggest that mGluRs couple to nearby protein synthesis machinery to homosynaptically regulate an intermediate phase of LTP dependent on new proteins made from pre-existing mRNA.
Figures
Similar articles
-
Priming of long-term potentiation induced by activation of metabotropic glutamate receptors coupled to phospholipase C.Hippocampus. 1998;8(2):160-70. doi: 10.1002/(SICI)1098-1063(1998)8:2<160::AID-HIPO8>3.0.CO;2-P. Hippocampus. 1998. PMID: 9572722
-
Evidence for involvement of group II/III metabotropic glutamate receptors in NMDA receptor-independent long-term potentiation in area CA1 of rat hippocampus.J Neurophysiol. 1999 Dec;82(6):2956-69. doi: 10.1152/jn.1999.82.6.2956. J Neurophysiol. 1999. PMID: 10601432
-
Selective induction of metabotropic glutamate receptor 1- and metabotropic glutamate receptor 5-dependent chemical long-term potentiation at oriens/alveus interneuron synapses of mouse hippocampus.Neuroscience. 2008 Jan 2;151(1):28-42. doi: 10.1016/j.neuroscience.2007.09.071. Epub 2007 Oct 11. Neuroscience. 2008. PMID: 18035501
-
Comparing the role of metabotropic glutamate receptors in long-term potentiation and in learning and memory.Prog Neuropsychopharmacol Biol Psychiatry. 1996 Jul;20(5):761-89. doi: 10.1016/0278-5846(96)00058-9. Prog Neuropsychopharmacol Biol Psychiatry. 1996. PMID: 8870063 Review.
-
Metabotropic glutamate receptors: electrophysiological properties and role in plasticity.Brain Res Brain Res Rev. 1999 Jan;29(1):83-120. doi: 10.1016/s0165-0173(98)00050-2. Brain Res Brain Res Rev. 1999. PMID: 9974152 Review.
Cited by
-
Loss of α1,6-Fucosyltransferase Decreases Hippocampal Long Term Potentiation: IMPLICATIONS FOR CORE FUCOSYLATION IN THE REGULATION OF AMPA RECEPTOR HETEROMERIZATION AND CELLULAR SIGNALING.J Biol Chem. 2015 Jul 10;290(28):17566-75. doi: 10.1074/jbc.M114.579938. Epub 2015 May 15. J Biol Chem. 2015. PMID: 25979332 Free PMC article.
-
The Role of mGlu Receptors in Hippocampal Plasticity Deficits in Neurological and Psychiatric Disorders: Implications for Allosteric Modulators as Novel Therapeutic Strategies.Curr Neuropharmacol. 2016;14(5):455-73. doi: 10.2174/1570159x13666150421003225. Curr Neuropharmacol. 2016. PMID: 27296640 Free PMC article. Review.
-
In vitro stretch injury induces time- and severity-dependent alterations of STEP phosphorylation and proteolysis in neurons.J Neurotrauma. 2012 Jul 1;29(10):1982-98. doi: 10.1089/neu.2011.2253. Epub 2012 Jun 25. J Neurotrauma. 2012. PMID: 22435660 Free PMC article.
-
Persistent phosphorylation by protein kinase Mzeta maintains late-phase long-term potentiation.J Neurosci. 2005 Feb 23;25(8):1979-84. doi: 10.1523/JNEUROSCI.5132-04.2005. J Neurosci. 2005. PMID: 15728837 Free PMC article.
-
Protein synthesis in the dendrite.Philos Trans R Soc Lond B Biol Sci. 2002 Apr 29;357(1420):521-9. doi: 10.1098/rstb.2001.0887. Philos Trans R Soc Lond B Biol Sci. 2002. PMID: 12028789 Free PMC article. Review.
References
-
- Abraham WC, Bear MF. Metaplasticity: the plasticity of synaptic plasticity. Trends Neurosci. 1996;19:126–130. - PubMed
-
- Abraham WC, Otani S. Macromolecules and the maintenance of long-term potentiation. In: Morrell F, editor. Kindling and synaptic plasticity. The legacy of Graham Goddard. Birkhauser; Boston: 1991. pp. 92–109.
-
- Abraham WC, Tate WP. Metaplasticity: a new vista across the field of synaptic plasticity. Prog Neurobiol. 1997;52:303–323. - PubMed
-
- Abraham WC, Demmer J, Richardson C, Williams J, Lawlor P, Mason SE, Tate WP, Dragunow M. Correlations between immediate early gene induction and the persistence of long-term potentiation. Neuroscience. 1993;56:717–727. - PubMed
-
- Bashir ZI, Bortolotto ZA, Davies CH, Berretta N, Irving A, Seal AJ, Henley JM, Jane DE, Watkins JC, Collingridge GA. Induction of LTP in the hippocampus needs synaptic activation of glutamate metabotropic receptors. Nature. 1993;363:347–350. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
