Atm and Bax cooperate in ionizing radiation-induced apoptosis in the central nervous system
- PMID: 10639175
- PMCID: PMC15426
- DOI: 10.1073/pnas.97.2.889
Atm and Bax cooperate in ionizing radiation-induced apoptosis in the central nervous system
Abstract
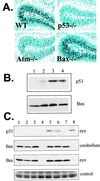
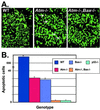
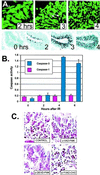
Ataxia-telangiectasia is a hereditary multisystemic disease resulting from mutations of ataxia telangiectasia, mutated (ATM) and is characterized by neurodegeneration, cancer, immune defects, and hypersensitivity to ionizing radiation. The molecular details of ATM function in the nervous system are unclear, although the neurological lesion in ataxia-telangiectasia becomes apparent early in life, suggesting a developmental origin. The central nervous system (CNS) of Atm-null mice shows a pronounced defect in apoptosis induced by genotoxic stress, suggesting ATM functions to eliminate neurons with excessive genomic damage. Here, we report that the death effector Bax is required for a large proportion of Atm-dependent apoptosis in the developing CNS after ionizing radiation (IR). Although many of the same regions of the CNS in both Bax-/- and Atm-/- mice were radioresistant, mice nullizygous for both Bax and Atm showed additional reduction in IR-induced apoptosis in the CNS. Therefore, although the major IR-induced apoptotic pathway in the CNS requires Atm and Bax, a p53-dependent collateral pathway exists that has both Atm- and Bax-independent branches. Further, Atm- and Bax-dependent apoptosis in the CNS also required caspase-3 activation. These data implicate Bax and caspase-3 as death effectors in neurodegenerative pathways.
Figures


Similar articles
-
Ataxia telangiectasia mutated-dependent apoptosis after genotoxic stress in the developing nervous system is determined by cellular differentiation status.J Neurosci. 2001 Sep 1;21(17):6687-93. doi: 10.1523/JNEUROSCI.21-17-06687.2001. J Neurosci. 2001. PMID: 11517258 Free PMC article.
-
Requirement for Atm in ionizing radiation-induced cell death in the developing central nervous system.Science. 1998 May 15;280(5366):1089-91. doi: 10.1126/science.280.5366.1089. Science. 1998. PMID: 9582124
-
ATM dependent apoptosis in the nervous system.Apoptosis. 2000 Dec;5(6):523-9. doi: 10.1023/a:1009637512917. Apoptosis. 2000. PMID: 11303911 Review.
-
The catalytic subunit of DNA-dependent protein kinase selectively regulates p53-dependent apoptosis but not cell-cycle arrest.Proc Natl Acad Sci U S A. 2000 Feb 15;97(4):1584-8. doi: 10.1073/pnas.97.4.1584. Proc Natl Acad Sci U S A. 2000. PMID: 10677503 Free PMC article.
-
Radiosensitivity and oxidative signalling in ataxia telangiectasia: an update.Radiother Oncol. 1998 May;47(2):113-23. doi: 10.1016/s0167-8140(98)00027-9. Radiother Oncol. 1998. PMID: 9683357 Review.
Cited by
-
Ataxia telangiectasia mutated is essential during adult neurogenesis.Genes Dev. 2001 Mar 1;15(5):554-66. doi: 10.1101/gad.869001. Genes Dev. 2001. PMID: 11238376 Free PMC article.
-
Mechanisms of chemotherapy-induced human ovarian aging: double strand DNA breaks and microvascular compromise.Aging (Albany NY). 2011 Aug;3(8):782-93. doi: 10.18632/aging.100363. Aging (Albany NY). 2011. PMID: 21869459 Free PMC article.
-
p53- and Bax-mediated apoptosis in injured rat spinal cord.Neurochem Res. 2011 Nov;36(11):2063-74. doi: 10.1007/s11064-011-0530-2. Epub 2011 Jul 7. Neurochem Res. 2011. PMID: 21748659
-
Defective Mitochondrial Dynamics and Protein Degradation Pathways Underlie Cadmium-Induced Neurotoxicity and Cell Death in Huntington's Disease Striatal Cells.Int J Mol Sci. 2023 Apr 13;24(8):7178. doi: 10.3390/ijms24087178. Int J Mol Sci. 2023. PMID: 37108341 Free PMC article.
-
Silencing Egr1 Attenuates Radiation-Induced Apoptosis in Normal Tissues while Killing Cancer Cells and Delaying Tumor Growth.Mol Cancer Ther. 2015 Oct;14(10):2343-52. doi: 10.1158/1535-7163.MCT-14-1051. Epub 2015 Jul 23. Mol Cancer Ther. 2015. PMID: 26206332 Free PMC article.
References
-
- Sedgwick R P, Boder E. In: Handbook of Clinical Neurology. Vinken P, Bruyn G, Klawans H, editors. Vol. 60. New York: Elsevier; 1991. pp. 347–423.
-
- Lavin M F, Shiloh Y. Annu Rev Immunol. 1997;15:177–202. - PubMed
-
- Soares H D, Morgan J I, McKinnon P J. Neuroscience. 1998;86:1045–1054. - PubMed
-
- Herzog K H, Chong M J, Kapsetaki M, Morgan J I, McKinnon P J. Science. 1998;280:1089–1091. - PubMed
-
- Adams J M, Cory S. Science. 1998;281:1322–1326. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

