Selective alterations in biosynthetic and endocytic protein traffic in Madin-Darby canine kidney epithelial cells expressing mutants of the small GTPase Rac1
- PMID: 10637309
- PMCID: PMC14775
- DOI: 10.1091/mbc.11.1.287
Selective alterations in biosynthetic and endocytic protein traffic in Madin-Darby canine kidney epithelial cells expressing mutants of the small GTPase Rac1
Abstract
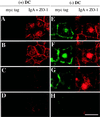
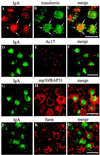
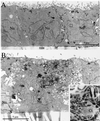
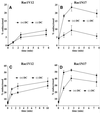
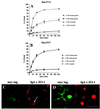
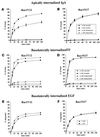
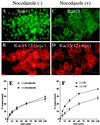
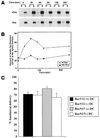
Madin-Darby canine kidney (MDCK) cells expressing constitutively active Rac1 (Rac1V12) accumulate a large central aggregate of membranes beneath the apical membrane that contains filamentous actin, Rac1V12, rab11, and the resident apical membrane protein GP-135. To examine the roles of Rac1 in membrane traffic and the formation of this aggregate, we analyzed endocytic and biosynthetic trafficking pathways in MDCK cells expressing Rac1V12 and dominant inactive Rac1 (Rac1N17). Rac1V12 expression decreased the rates of apical and basolateral endocytosis, whereas Rac1N17 expression increased those rates from both membrane domains. Basolateral-to-apical transcytosis of immunoglobulin A (IgA) (a ligand for the polymeric immunoglobulin receptor [pIgR]), apical recycling of pIgR-IgA, and accumulation of newly synthesized GP-135 at the apical plasma membrane were all decreased in cells expressing Rac1V12. These effects of Rac1V12 on trafficking pathways to the apical membrane were the result of the delivery and trapping of these proteins in the central aggregate. In contrast to abnormalities in apical trafficking events, basolateral recycling of transferrin, degradation of EGF internalized from the basolateral membrane, and delivery of newly synthesized pIgR from the Golgi to the basolateral membrane were all relatively unaffected by Rac1V12 expression. Rac1N17 expression had little or no effect on these postendocytic or biosynthetic trafficking pathways. These results show that in polarized MDCK cells activated Rac1 may regulate the rate of endocytosis from both membrane domains and that expression of dominant active Rac1V12 specifically alters postendocytic and biosynthetic membrane traffic directed to the apical, but not the basolateral, membrane.
Figures


Similar articles
-
Modulation of endocytic traffic in polarized Madin-Darby canine kidney cells by the small GTPase RhoA.Mol Biol Cell. 1999 Dec;10(12):4369-84. doi: 10.1091/mbc.10.12.4369. Mol Biol Cell. 1999. PMID: 10588664 Free PMC article.
-
Effects of regulated expression of mutant RhoA and Rac1 small GTPases on the development of epithelial (MDCK) cell polarity.J Cell Biol. 1998 Jul 13;142(1):85-100. doi: 10.1083/jcb.142.1.85. J Cell Biol. 1998. PMID: 9660865 Free PMC article.
-
Association of Rab25 and Rab11a with the apical recycling system of polarized Madin-Darby canine kidney cells.Mol Biol Cell. 1999 Jan;10(1):47-61. doi: 10.1091/mbc.10.1.47. Mol Biol Cell. 1999. PMID: 9880326 Free PMC article.
-
Endocytic traffic in polarized epithelial cells: role of the actin and microtubule cytoskeleton.Traffic. 2001 Mar;2(3):149-59. doi: 10.1034/j.1600-0854.2001.020301.x. Traffic. 2001. PMID: 11260520 Review.
-
Cargo sorting in the endocytic pathway: a key regulator of cell polarity and tissue dynamics.Cold Spring Harb Perspect Biol. 2014 Aug 14;6(10):a016899. doi: 10.1101/cshperspect.a016899. Cold Spring Harb Perspect Biol. 2014. PMID: 25125399 Free PMC article. Review.
Cited by
-
Cdc42-dependent modulation of tight junctions and membrane protein traffic in polarized Madin-Darby canine kidney cells.Mol Biol Cell. 2001 Aug;12(8):2257-74. doi: 10.1091/mbc.12.8.2257. Mol Biol Cell. 2001. PMID: 11514615 Free PMC article.
-
Differential localization of Rho GTPases in live cells: regulation by hypervariable regions and RhoGDI binding.J Cell Biol. 2001 Jan 8;152(1):111-26. doi: 10.1083/jcb.152.1.111. J Cell Biol. 2001. PMID: 11149925 Free PMC article.
-
Cdc42 and the phosphatidylinositol 3-kinase-Akt pathway are essential for PspC-mediated internalization of pneumococci by respiratory epithelial cells.J Biol Chem. 2009 Jul 17;284(29):19427-36. doi: 10.1074/jbc.M109.003442. Epub 2009 May 27. J Biol Chem. 2009. PMID: 19473971 Free PMC article.
-
Myelin biogenesis: vesicle transport in oligodendrocytes.Neurochem Res. 2002 Nov;27(11):1313-29. doi: 10.1023/a:1021667515030. Neurochem Res. 2002. PMID: 12512937 Review.
-
The non-catalytic carboxyl-terminal domain of ARFGAP1 regulates actin cytoskeleton reorganization by antagonizing the activation of Rac1.PLoS One. 2011 Apr 4;6(4):e18458. doi: 10.1371/journal.pone.0018458. PLoS One. 2011. PMID: 21483700 Free PMC article.
References
-
- Apodaca G, Aroeti B, Tang K, Mostov KE. Brefeldin-A inhibits the delivery of the polymeric immunoglobulin receptor to the basolateral surface of MDCK cells. J Biol Chem. 1993;268:20380–20385. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

