Critical role for CD8 in T cell receptor binding and activation by peptide/major histocompatibility complex multimers
- PMID: 10637277
- PMCID: PMC2195759
- DOI: 10.1084/jem.191.2.335
Critical role for CD8 in T cell receptor binding and activation by peptide/major histocompatibility complex multimers
Abstract
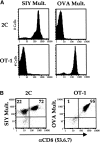
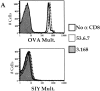
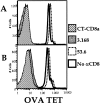
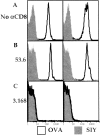
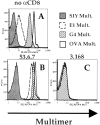
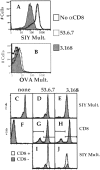
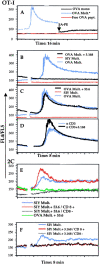
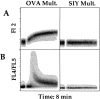
Recent data using MHC/peptide tetramers and dimers suggests that the T cell coreceptors, CD4 and CD8, although important for T cell activation, do not play a direct role in facilitating T cell receptor (TCR) binding to multivalent MHC/peptide ligands. Instead, a current model proposes that coreceptors are recruited only after a stable TCR-MHC/peptide complex has already formed and signaled. In contrast, we show using multimeric class I MHC/peptide ligands that CD8 plays a critical (in some cases obligatory) role in antigen-specific TCR binding. T cell activation, measured by calcium mobilization, was induced by multimeric but not monomeric ligands and also showed CD8 dependency. Our analysis using anti-CD8 antibodies revealed that binding to different epitopes of CD8 can either block or augment TCR-MHC/peptide interaction. These effects on TCR binding to high-affinity agonist ligands were even more pronounced when binding to multimeric low-affinity ligands, including TCR antagonists, was studied. Our data have important implications for the role of CD8 in TCR binding to MHC/peptide ligands and in T cell activation. In addition, our results argue against the view that multimeric MHC/peptide ligands bind directly and solely to the TCR; rather, our data highlight a pivotal contribution of CD8 for this association.
Figures

Similar articles
-
Critical role for CD8 in binding of MHC tetramers to TCR: CD8 antibodies block specific binding of human tumor-specific MHC-peptide tetramers to TCR.J Immunol. 2001 Jul 1;167(1):270-6. doi: 10.4049/jimmunol.167.1.270. J Immunol. 2001. PMID: 11418659
-
Glu227-->Lys substitution in the acidic loop of major histocompatibility complex class I alpha 3 domain distinguishes low avidity CD8 coreceptor and avidity-enhanced CD8 accessory functions.J Exp Med. 1996 Nov 1;184(5):1671-83. doi: 10.1084/jem.184.5.1671. J Exp Med. 1996. PMID: 8920857 Free PMC article.
-
CD8 T cells, like CD4 T cells, are triggered by multivalent engagement of TCRs by MHC-peptide ligands but not by monovalent engagement.J Immunol. 2006 Feb 1;176(3):1498-505. doi: 10.4049/jimmunol.176.3.1498. J Immunol. 2006. PMID: 16424178
-
Recognition surfaces of MHC class I.Immunol Rev. 1998 Jun;163:121-8. doi: 10.1111/j.1600-065x.1998.tb01191.x. Immunol Rev. 1998. PMID: 9700505 Review.
-
Interplay between the TCR/CD3 complex and CD4 or CD8 in the activation of cytotoxic T lymphocytes.Immunol Rev. 1989 Jun;109:119-41. doi: 10.1111/j.1600-065x.1989.tb00022.x. Immunol Rev. 1989. PMID: 2527803 Review.
Cited by
-
CD8 coreceptor engagement of MR1 enhances antigen responsiveness by human MAIT and other MR1-reactive T cells.J Exp Med. 2022 Sep 5;219(9):e20210828. doi: 10.1084/jem.20210828. Epub 2022 Aug 26. J Exp Med. 2022. PMID: 36018322 Free PMC article.
-
Effector-like CD8⁺ T cells in the memory population mediate potent protective immunity.Immunity. 2013 Jun 27;38(6):1250-60. doi: 10.1016/j.immuni.2013.05.009. Epub 2013 Jun 6. Immunity. 2013. PMID: 23746652 Free PMC article.
-
Memory generation and maintenance of CD8+ T cell function during viral persistence.J Immunol. 2007 Jul 1;179(1):141-53. doi: 10.4049/jimmunol.179.1.141. J Immunol. 2007. PMID: 17579032 Free PMC article.
-
Dependence of lymphopenia-induced T cell proliferation on the abundance of peptide/ MHC epitopes and strength of their interaction with T cell receptors.Proc Natl Acad Sci U S A. 2001 Feb 13;98(4):1728-33. doi: 10.1073/pnas.98.4.1728. Proc Natl Acad Sci U S A. 2001. PMID: 11172019 Free PMC article.
-
Two-stage cooperative T cell receptor-peptide major histocompatibility complex-CD8 trimolecular interactions amplify antigen discrimination.Immunity. 2011 Jan 28;34(1):13-23. doi: 10.1016/j.immuni.2010.12.017. Epub 2011 Jan 20. Immunity. 2011. PMID: 21256056 Free PMC article.
References
-
- Janeway C.A., Jr. The T cell receptor as a multicomponent signalling machineCD4/CD8 coreceptors and CD45 in T cell activation. Annu. Rev. Immunol. 1992;10:645–674. - PubMed
-
- Miceli M.C., Parnes J.R. Role of CD4 and CD8 in T cell activation and differentiation. Adv. Immunol. 1993;53:59–122. - PubMed
-
- Zamoyska R. CD4 and CD8modulators of T-cell receptor recognition of antigen and of immune responses? Curr. Opin. Immunol. 1998;10:82–87. - PubMed
-
- Eichmann K., Boyce N.W., Schmidt-Ullrich R., Jonsson J.I. Distinct functions of CD8(CD4) are utilized at different stages of T-lymphocyte differentiation. Immunol. Rev. 1989;109:39–75. - PubMed
-
- Luescher I.F., Vivier E., Layer A., Mahiou J., Godeau F., Malissen B., Romero P. CD8 modulation of T-cell antigen receptor-ligand interactions on living cytotoxic T lymphocytes. Nature. 1995;373:353–356. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

