Monocytes induce reversible focal changes in vascular endothelial cadherin complex during transendothelial migration under flow
- PMID: 10629229
- PMCID: PMC2156206
- DOI: 10.1083/jcb.148.1.203
Monocytes induce reversible focal changes in vascular endothelial cadherin complex during transendothelial migration under flow
Abstract
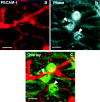
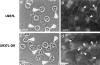
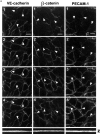
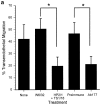
The vascular endothelial cell cadherin complex (VE-cadherin, alpha-, beta-, and gamma-catenin, and p120/p100) localizes to adherens junctions surrounding vascular endothelial cells and may play a critical role in the transendothelial migration of circulating blood leukocytes. Previously, we have reported that neutrophil adhesion to human umbilical vein endothelial cell (HUVEC) monolayers, under static conditions, results in a dramatic loss of the VE-cadherin complex. Subsequent studies by us and others (Moll, T., E. Dejana, and D. Vestweber. 1998. J. Cell Biol. 140:403-407) suggested that this phenomenon might reflect degradation by neutrophil proteases released during specimen preparation. We postulated that some form of disruption of the VE-cadherin complex might, nonetheless, be a physiological process during leukocyte transmigration. In the present study, the findings demonstrate a specific, localized effect of migrating leukocytes on the VE-cadherin complex in cytokine-activated HUVEC monolayers. Monocytes and in vitro differentiated U937 cells induce focal loss in the staining of VE-cadherin, alpha-catenin, beta-catenin, and plakoglobin during transendothelial migration under physiological flow conditions. These events are inhibited by antibodies that prevent transendothelial migration and are reversed following transmigration. Together, these data suggest that an endothelial-dependent step of transient and focal disruption of the VE-cadherin complex occurs during leukocyte transmigration.
Figures



Similar articles
-
Dynamics of vascular endothelial-cadherin and beta-catenin localization by vascular endothelial growth factor-induced angiogenesis in human umbilical vein cells.Exp Cell Res. 2002 Nov 1;280(2):159-68. doi: 10.1006/excr.2002.5636. Exp Cell Res. 2002. PMID: 12413882
-
Neutrophils induce sequential focal changes in endothelial adherens junction components: role of elastase.Microcirculation. 2003 Apr;10(2):205-20. doi: 10.1038/sj.mn.7800185. Microcirculation. 2003. PMID: 12700588
-
The presence of alpha-catenin in the VE-cadherin complex is required for efficient transendothelial migration of leukocytes.Int J Biol Sci. 2009 Nov 9;5(7):695-705. doi: 10.7150/ijbs.5.695. Int J Biol Sci. 2009. PMID: 19918298 Free PMC article.
-
Changes in the distribution of LFA-1, catenins, and F-actin during transendothelial migration of monocytes in culture.J Cell Sci. 1997 Nov;110 ( Pt 22):2807-18. doi: 10.1242/jcs.110.22.2807. J Cell Sci. 1997. PMID: 9427289
-
The role of adherens junctions and VE-cadherin in the control of vascular permeability.J Cell Sci. 2008 Jul 1;121(Pt 13):2115-22. doi: 10.1242/jcs.017897. J Cell Sci. 2008. PMID: 18565824 Review.
Cited by
-
Proteinase 3 contributes to transendothelial migration of NB1-positive neutrophils.J Immunol. 2012 Mar 1;188(5):2419-26. doi: 10.4049/jimmunol.1102540. Epub 2012 Jan 20. J Immunol. 2012. PMID: 22266279 Free PMC article.
-
Human cytomegalovirus (HCMV) infection of endothelial cells promotes naive monocyte extravasation and transfer of productive virus to enhance hematogenous dissemination of HCMV.J Virol. 2006 Dec;80(23):11539-55. doi: 10.1128/JVI.01016-06. Epub 2006 Sep 20. J Virol. 2006. PMID: 16987970 Free PMC article.
-
Mechanisms of leukocyte transendothelial migration.Annu Rev Pathol. 2011;6:323-44. doi: 10.1146/annurev-pathol-011110-130224. Annu Rev Pathol. 2011. PMID: 21073340 Free PMC article. Review.
-
Mechanisms of transendothelial migration of leukocytes.Circ Res. 2009 Jul 31;105(3):223-30. doi: 10.1161/CIRCRESAHA.109.200717. Circ Res. 2009. PMID: 19644057 Free PMC article. Review.
-
Diabetic retinopathy and inflammation: novel therapeutic targets.Middle East Afr J Ophthalmol. 2012 Jan;19(1):52-9. doi: 10.4103/0974-9233.92116. Middle East Afr J Ophthalmol. 2012. PMID: 22346115 Free PMC article.
References
-
- Ali J., Liao F., Martens E., Muller W.A. Vascular endothelial cadherin (VE-cadherin)cloning and role in endothelial cell–cell adhesion. Microcirculation. 1997;4:267–277. - PubMed
-
- Allport J.R., Ding H., Collins T., Gerritsen M.E., Luscinskas F.W. Endothelial-dependent mechanisms regulate leukocyte transmigrationa process involving the proteasome and disruption of the vascular endothelial–cadherin complex at endothelial cell-to-cell junctions. J. Exp. Med. 1997;186:517–527. - PMC - PubMed
-
- Berman M.E., Muller W.A. Ligation of platelet/endothelial cell adhesion molecule-1 (PECAM-1/CD31) on monocytes and neutrophils increases binding capacity of leukocyte CR3 (CD11b/CD18) J. Immunol. 1995;154:299–307. - PubMed
-
- Breviario F., Caveda L., Corada M., Martin-Padura I., Navarro P., Golay J., Introna M., Gulino D., Lampugnani M.G., Dejana E. Functional properties of human vascular endothelial cadherin (7B4/cadherin-5), an endothelium-specific cadherin. Arterioscler. Thromb. Vasc. Biol. 1995;15:1229–1239. - PubMed
-
- Burns A.R., Walker D.C., Brown E.S., Thurmon L.T., Bowden R.A., Keese C.R., Simon S.I., Entman M.L., Smith C.W. Neutrophil transendothelial migration is independent of tight junctions and occurs preferentially at tricellular corners. J. Immunol. 1997;159:2893–2903. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

