Orf virus encodes a novel secreted protein inhibitor of granulocyte-macrophage colony-stimulating factor and interleukin-2
- PMID: 10627542
- PMCID: PMC111466
- DOI: 10.1128/jvi.74.3.1313-1320.2000
Orf virus encodes a novel secreted protein inhibitor of granulocyte-macrophage colony-stimulating factor and interleukin-2
Abstract
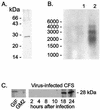
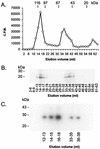
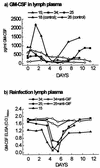
The parapoxvirus orf virus encodes a novel soluble protein inhibitor of ovine granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin-2 (IL-2). The GM-CSF- and IL-2-inhibitory factor (GIF) gene was expressed as an intermediate-late viral gene in orf virus-infected cells. GIF formed homodimers and tetramers in solution, and it bound ovine GM-CSF with a K(d) of 369 pM and ovine IL-2 with a K(d) of 1.04 nM. GIF did not bind human GM-CSF or IL-2 in spite of the fact that orf virus is a human pathogen. GIF was detected in afferent lymph plasma draining the skin site of orf virus reinfection and was associated with reduced levels of lymph GM-CSF. GIF expression by orf virus indicates that GM-CSF and IL-2 are important in host antiviral immunity.
Figures



Similar articles
-
Conservation and variation of the parapoxvirus GM-CSF-inhibitory factor (GIF) proteins.J Gen Virol. 2009 Apr;90(Pt 4):970-977. doi: 10.1099/vir.0.006692-0. Epub 2009 Mar 4. J Gen Virol. 2009. PMID: 19264672
-
Glycosylation, disulfide bond formation, and the presence of a WSXWS-like motif in the orf virus GIF protein are critical for maintaining the integrity of Binding to ovine granulocyte-macrophage colony-stimulating factor and interleukin-2.J Virol. 2005 Sep;79(17):11205-13. doi: 10.1128/JVI.79.17.11205-11213.2005. J Virol. 2005. PMID: 16103172 Free PMC article.
-
Immunomodulation by virulence proteins of the parapoxvirus orf virus.Vet Immunol Immunopathol. 1999 Dec 15;72(1-2):81-6. doi: 10.1016/s0165-2427(99)00119-1. Vet Immunol Immunopathol. 1999. PMID: 10614496 Review.
-
Orf virus immuno-modulation and the host immune response.Vet Immunol Immunopathol. 2002 Sep 10;87(3-4):395-9. doi: 10.1016/s0165-2427(02)00087-9. Vet Immunol Immunopathol. 2002. PMID: 12072264
-
Ovine diseases. Orf.Vet Res. 1998 May-Aug;29(3-4):311-26. Vet Res. 1998. PMID: 9689744 Review.
Cited by
-
Genome of crocodilepox virus.J Virol. 2006 May;80(10):4978-91. doi: 10.1128/JVI.80.10.4978-4991.2006. J Virol. 2006. PMID: 16641289 Free PMC article.
-
Cyclophilin B facilitates the replication of Orf virus.Virol J. 2017 Jun 15;14(1):114. doi: 10.1186/s12985-017-0781-x. Virol J. 2017. PMID: 28619100 Free PMC article.
-
Orf virus interferes with MHC class I surface expression by targeting vesicular transport and Golgi.BMC Vet Res. 2012 Jul 18;8:114. doi: 10.1186/1746-6148-8-114. BMC Vet Res. 2012. PMID: 22809544 Free PMC article.
-
Structural basis of GM-CSF and IL-2 sequestration by the viral decoy receptor GIF.Nat Commun. 2016 Nov 7;7:13228. doi: 10.1038/ncomms13228. Nat Commun. 2016. PMID: 27819269 Free PMC article.
-
Construction of a Triple-Gene Deletion Mutant of Orf Virus and Evaluation of Its Safety, Immunogenicity and Protective Efficacy.Vaccines (Basel). 2023 Apr 28;11(5):909. doi: 10.3390/vaccines11050909. Vaccines (Basel). 2023. PMID: 37243014 Free PMC article.
References
-
- Alcamí A, Smith G L. A soluble receptor for interleukin-1β encoded by vaccinia virus: a novel mechanism of virus modulation of the host response to infection. Cell. 1992;71:153–167. - PubMed
-
- Brown C B, Beaudry P, Laing T D, Shoemaker S, Kaushansky K. In vitro characterisation of the human recombinant soluble granulocyte-macrophage colony-stimulating factor receptor. Blood. 1995;85:1488–1495. - PubMed
-
- Brown C B, Pihl C E, Murray E W. Oligomerization of the soluble granulocyte-macrophage colony-stimulating factor receptor: identification of the functional ligand-binding species. Cytokine. 1997;9:219–225. - PubMed
-
- Bujdoso R, Sargan D, Williamson M, McConnell I. Cloning of a cDNA encoding the ovine interleukin-2 receptor 55-kDa protein, CD25. Gene. 1992;113:283–284. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

