A recombinant human immunodeficiency virus type 1 envelope glycoprotein complex stabilized by an intermolecular disulfide bond between the gp120 and gp41 subunits is an antigenic mimic of the trimeric virion-associated structure
- PMID: 10623724
- PMCID: PMC111582
- DOI: 10.1128/jvi.74.2.627-643.2000
A recombinant human immunodeficiency virus type 1 envelope glycoprotein complex stabilized by an intermolecular disulfide bond between the gp120 and gp41 subunits is an antigenic mimic of the trimeric virion-associated structure
Abstract
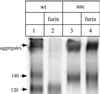
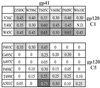
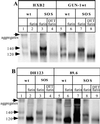
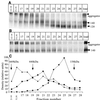
The few antibodies that can potently neutralize human immunodeficiency virus type 1 (HIV-1) recognize the limited number of envelope glycoprotein epitopes exposed on infectious virions. These native envelope glycoprotein complexes comprise three gp120 subunits noncovalently and weakly associated with three gp41 moieties. The individual subunits induce neutralizing antibodies inefficiently but raise many nonneutralizing antibodies. Consequently, recombinant envelope glycoproteins do not elicit strong antiviral antibody responses, particularly against primary HIV-1 isolates. To try to develop recombinant proteins that are better antigenic mimics of the native envelope glycoprotein complex, we have introduced a disulfide bond between the C-terminal region of gp120 and the immunodominant segment of the gp41 ectodomain. The resulting gp140 protein is processed efficiently, producing a properly folded envelope glycoprotein complex. The association of gp120 with gp41 is now stabilized by the supplementary intermolecular disulfide bond, which forms with approximately 50% efficiency. The gp140 protein has antigenic properties which resemble those of the virion-associated complex. This type of gp140 protein may be worth evaluating for immunogenicity as a component of a multivalent HIV-1 vaccine.
Figures


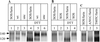




Similar articles
-
Stabilization of the soluble, cleaved, trimeric form of the envelope glycoprotein complex of human immunodeficiency virus type 1.J Virol. 2002 Sep;76(17):8875-89. doi: 10.1128/jvi.76.17.8875-8889.2002. J Virol. 2002. PMID: 12163607 Free PMC article.
-
Variable-loop-deleted variants of the human immunodeficiency virus type 1 envelope glycoprotein can be stabilized by an intermolecular disulfide bond between the gp120 and gp41 subunits.J Virol. 2000 Jun;74(11):5091-100. doi: 10.1128/jvi.74.11.5091-5100.2000. J Virol. 2000. PMID: 10799583 Free PMC article.
-
Characterization of stable, soluble trimers containing complete ectodomains of human immunodeficiency virus type 1 envelope glycoproteins.J Virol. 2000 Jun;74(12):5716-25. doi: 10.1128/jvi.74.12.5716-5725.2000. J Virol. 2000. PMID: 10823881 Free PMC article.
-
Relevance of the antibody response against human immunodeficiency virus type 1 envelope to vaccine design.Immunol Lett. 1997 Jun 1;57(1-3):105-12. doi: 10.1016/s0165-2478(97)00043-6. Immunol Lett. 1997. Corrected and republished in: Immunol Lett. 1997 Jul;58(2):125-32. doi: 10.1016/s0165-2478(97)00109-0. PMID: 9232434 Corrected and republished. Review.
-
Erratum to "Relevance of the antibody response against human immunodeficiency virus type 1 envelope to vaccine design".Immunol Lett. 1997 Jul;58(2):125-32. doi: 10.1016/s0165-2478(97)00109-0. Immunol Lett. 1997. PMID: 9271324 Review.
Cited by
-
Structure-based design of a soluble human cytomegalovirus glycoprotein B antigen stabilized in a prefusion-like conformation.Proc Natl Acad Sci U S A. 2024 Sep 10;121(37):e2404250121. doi: 10.1073/pnas.2404250121. Epub 2024 Sep 4. Proc Natl Acad Sci U S A. 2024. PMID: 39231203 Free PMC article.
-
mRNA lipid nanoparticles expressing cell-surface cleavage independent HIV Env trimers elicit autologous tier-2 neutralizing antibodies.Front Immunol. 2024 Jul 25;15:1426232. doi: 10.3389/fimmu.2024.1426232. eCollection 2024. Front Immunol. 2024. PMID: 39119336 Free PMC article.
-
Impact of glycan depletion, glycan debranching and increased glycan charge on HIV-1 neutralization sensitivity and immunogenicity.Glycobiology. 2024 Sep 30;34(11):cwae063. doi: 10.1093/glycob/cwae063. Glycobiology. 2024. PMID: 39115361
-
Design of soluble HIV-1 envelope trimers free of covalent gp120-gp41 bonds with prevalent native-like conformation.Cell Rep. 2024 Aug 27;43(8):114518. doi: 10.1016/j.celrep.2024.114518. Epub 2024 Jul 18. Cell Rep. 2024. PMID: 39028623 Free PMC article.
-
Production and Immunogenicity of FeLV Gag-Based VLPs Exposing a Stabilized FeLV Envelope Glycoprotein.Viruses. 2024 Jun 19;16(6):987. doi: 10.3390/v16060987. Viruses. 2024. PMID: 38932278 Free PMC article.
References
-
- Abacioglu Y H, Fouts T R, Laman J D, Claassen E, Pincus S H, Moore J P, Roby C A, Kamin-Lewis R, Lewis G K. Epitope mapping and topology of baculovirus expressed HIV-1 gp160 determined with a panel of murine monoclonal antibodies. AIDS Res Hum Retroviruses. 1994;10:371–381. - PubMed
-
- Allaway G P, Davis-Bruno K L, Beaudry G A, Garcia E B, Wong E L, Ryder A M, Hasel K W, Gauduin M-C, Koup R A, McDougal J S, Maddon P J. Expression and characterization of CD4lgG2, a novel heterotetramer which neutralizes primary HIV-1 isolates. AIDS Res Hum Retroviruses. 1995;11:533–540. - PubMed
-
- Barnett S W, Rajasekar S, Legg H, Doe B, Fuller D H, Haynes J R, Walker C M, Steimer K S. Vaccination with HIV-1 gp120 DNA induces immune responses that are boosted by a recombinant gp120 protein subunit. Vaccine. 1997;15:869–873. - PubMed
-
- Belshe R B, Gorse G J, Mulligan M J, Evans T G, Keefer M C, Excler J-L, Duliege A M, Tartaglia J, Cox W I, McNamara J, Hwang K-L, Bradney A, Montefiori D C, Weinhold K J. Induction of immune responses to HIV-1 by canarypox virus (ALVAC) HIV-1 and gp120 SF-2 recombinant vaccines in uninfected volunteers. AIDS. 1998;12:2407–2415. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

