NMR solution structure of the human prion protein
- PMID: 10618385
- PMCID: PMC26630
- DOI: 10.1073/pnas.97.1.145
NMR solution structure of the human prion protein
Abstract
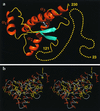
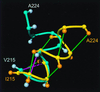
The NMR structures of the recombinant human prion protein, hPrP(23-230), and two C-terminal fragments, hPrP(90-230) and hPrP(121-230), include a globular domain extending from residues 125-228, for which a detailed structure was obtained, and an N-terminal flexibly disordered "tail." The globular domain contains three alpha-helices comprising the residues 144-154, 173-194, and 200-228 and a short anti-parallel beta-sheet comprising the residues 128-131 and 161-164. Within the globular domain, three polypeptide segments show increased structural disorder: i.e., a loop of residues 167-171, the residues 187-194 at the end of helix 2, and the residues 219-228 in the C-terminal part of helix 3. The local conformational state of the polypeptide segments 187-193 in helix 2 and 219-226 in helix 3 is measurably influenced by the length of the N-terminal tail, with the helical states being most highly populated in hPrP(23-230). When compared with the previously reported structures of the murine and Syrian hamster prion proteins, the length of helix 3 coincides more closely with that in the Syrian hamster protein whereas the disordered loop 167-171 is shared with murine PrP. These species variations of local structure are in a surface area of the cellular form of PrP that has previously been implicated in intermolecular interactions related both to the species barrier for infectious transmission of prion disease and to immune reactions.
Figures

Similar articles
-
NMR structure of the human doppel protein.J Mol Biol. 2003 Mar 7;326(5):1549-57. doi: 10.1016/s0022-2836(02)01471-7. J Mol Biol. 2003. PMID: 12595265
-
NMR structure of the bovine prion protein.Proc Natl Acad Sci U S A. 2000 Jul 18;97(15):8334-9. doi: 10.1073/pnas.97.15.8334. Proc Natl Acad Sci U S A. 2000. PMID: 10899999 Free PMC article.
-
NMR structures of three single-residue variants of the human prion protein.Proc Natl Acad Sci U S A. 2000 Jul 18;97(15):8340-5. doi: 10.1073/pnas.97.15.8340. Proc Natl Acad Sci U S A. 2000. PMID: 10900000 Free PMC article.
-
Molecular structures of amyloid and prion fibrils: consensus versus controversy.Acc Chem Res. 2013 Jul 16;46(7):1487-96. doi: 10.1021/ar300282r. Epub 2013 Jan 7. Acc Chem Res. 2013. PMID: 23294335 Free PMC article. Review.
-
The prion protein: Structural features and related toxic peptides.Chem Biol Drug Des. 2006 Sep;68(3):139-47. doi: 10.1111/j.1747-0285.2006.00427.x. Chem Biol Drug Des. 2006. PMID: 17062011 Review.
Cited by
-
Improving Internal Peptide Dynamics in the Coarse-Grained MARTINI Model: Toward Large-Scale Simulations of Amyloid- and Elastin-like Peptides.J Chem Theory Comput. 2012 May 8;8(5):1774-1785. doi: 10.1021/ct200876v. Epub 2012 Mar 26. J Chem Theory Comput. 2012. PMID: 22582033 Free PMC article.
-
A novel mutation I215V in the PRNP gene associated with Creutzfeldt-Jakob and Alzheimer's diseases in three patients with divergent clinical phenotypes.J Neurol. 2013 Jan;260(1):77-84. doi: 10.1007/s00415-012-6588-1. Epub 2012 Jul 5. J Neurol. 2013. PMID: 22763467
-
The effect of β2-α2 loop mutation on amyloidogenic properties of the prion protein.FEBS Lett. 2013 Sep 17;587(18):2918-23. doi: 10.1016/j.febslet.2013.07.023. Epub 2013 Jul 24. FEBS Lett. 2013. PMID: 23892077 Free PMC article.
-
Structurally distinct external solvent-exposed domains drive replication of major human prions.PLoS Pathog. 2021 Jun 17;17(6):e1009642. doi: 10.1371/journal.ppat.1009642. eCollection 2021 Jun. PLoS Pathog. 2021. PMID: 34138981 Free PMC article.
-
Changing a single amino acid in the N-terminus of murine PrP alters TSE incubation time across three species barriers.EMBO J. 2001 Sep 17;20(18):5070-8. doi: 10.1093/emboj/20.18.5070. EMBO J. 2001. PMID: 11566872 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

