Activation of a cell entry pathway common to type C mammalian retroviruses by soluble envelope fragments
- PMID: 10590117
- PMCID: PMC111539
- DOI: 10.1128/jvi.74.1.295-304.2000
Activation of a cell entry pathway common to type C mammalian retroviruses by soluble envelope fragments
Abstract
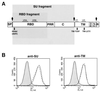
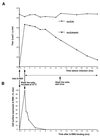
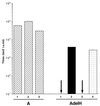
Mutations that negatively or positively affect the fusion properties of murine leukemia viruses (MLVs) have been found within all subdomains of their SU (surface) and TM (transmembrane) envelope units. Yet, the interrelations between these different regions of the envelope complex during the cell entry process are still elusive. Deletion of the histidine residue of the conserved PHQV motif at the amino terminus of the amphotropic or the ecotropic MLV SU resulted in the AdelH or the MOdelH fusion-defective mutant envelope, respectively. These delH mutant envelopes are incorporated on retroviral particles at normal densities and normally mediate virion binding to cells expressing the retroviral receptors. However, both their cell-cell and virus-cell fusogenicities were fully prevented at an early postbinding stage. We show here that the fusion defect of AdelH or MOdelH envelopes was also almost completely reverted by providing either soluble SU or a polypeptide encompassing the receptor-binding domain (RBD) to the target cells, provided that the integrity of the amino-terminal end of either polypeptide was preserved. Restoration of delH envelope fusogenicity was caused by activation of the target cells via specific interaction of the latter polypeptides with the retrovirus receptor rather than by their association with the delH envelope complexes. Moreover crossactivation of the target cells, leading to fusion activation of AdelH or MOdelH envelopes, was achieved by polypeptides containing various type C mammalian retrovirus RBDs, irrespective of the type of entry-defective glycoprotein that was used for infection. Our results indicate that although they recognize different receptors for binding to the cell surface, type C mammalian retroviruses use a common entry pathway which is activated by a conserved feature of their envelope glycoproteins.
Figures
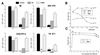
Similar articles
-
A proline-rich motif downstream of the receptor binding domain modulates conformation and fusogenicity of murine retroviral envelopes.J Virol. 1998 Dec;72(12):9955-65. doi: 10.1128/JVI.72.12.9955-9965.1998. J Virol. 1998. PMID: 9811733 Free PMC article.
-
Relationship between SU subdomains that regulate the receptor-mediated transition from the native (fusion-inhibited) to the fusion-active conformation of the murine leukemia virus glycoprotein.J Virol. 2002 Oct;76(19):9673-85. doi: 10.1128/jvi.76.19.9673-9685.2002. J Virol. 2002. PMID: 12208946 Free PMC article.
-
Activation of membrane fusion by murine leukemia viruses is controlled in cis or in trans by interactions between the receptor-binding domain and a conserved disulfide loop of the carboxy terminus of the surface glycoprotein.J Virol. 2001 Apr;75(8):3685-95. doi: 10.1128/JVI.75.8.3685-3695.2001. J Virol. 2001. PMID: 11264358 Free PMC article.
-
The HTLV-I envelope glycoproteins: structure and functions.J Acquir Immune Defic Syndr Hum Retrovirol. 1996;13 Suppl 1:S85-91. doi: 10.1097/00042560-199600001-00015. J Acquir Immune Defic Syndr Hum Retrovirol. 1996. PMID: 8797709 Review.
-
Cell surface receptors for gammaretroviruses.Curr Top Microbiol Immunol. 2003;281:29-106. doi: 10.1007/978-3-642-19012-4_2. Curr Top Microbiol Immunol. 2003. PMID: 12932075 Review.
Cited by
-
Comprehensive mutational analysis of the Moloney murine leukemia virus envelope protein.J Virol. 2001 Dec;75(23):11851-62. doi: 10.1128/JVI.75.23.11851-11862.2001. J Virol. 2001. PMID: 11689666 Free PMC article.
-
Murine leukemia virus (MLV) replication monitored with fluorescent proteins.Virol J. 2004 Dec 20;1:14. doi: 10.1186/1743-422X-1-14. Virol J. 2004. PMID: 15610559 Free PMC article.
-
Sequences in the cytoplasmic tail of the gibbon ape leukemia virus envelope protein that prevent its incorporation into lentivirus vectors.J Virol. 2001 May;75(9):4129-38. doi: 10.1128/JVI.75.9.4129-4138.2001. J Virol. 2001. PMID: 11287562 Free PMC article.
-
Distinct mechanisms of neutralization by monoclonal antibodies specific for sites in the N-terminal or C-terminal domain of murine leukemia virus SU.J Virol. 2003 Apr;77(7):3993-4003. doi: 10.1128/jvi.77.7.3993-4003.2003. J Virol. 2003. PMID: 12634359 Free PMC article.
-
Unique Structure and Distinctive Properties of the Ancient and Ubiquitous Gamma-Type Envelope Glycoprotein.Viruses. 2023 Jan 18;15(2):274. doi: 10.3390/v15020274. Viruses. 2023. PMID: 36851488 Free PMC article. Review.
References
-
- Chesebro B, Wehrly K, Cloyd M, Britt W, Portis J, Collins J, Nishio J. Characterization of mouse monoclonal antibodies specific for Friend murine leukemia virus-induced erythroleukemia cells: Friend-specific and FMR-specific antigens. Virology. 1981;112:131–144. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

