Specific therapy regimes could lead to long-term immunological control of HIV
- PMID: 10588728
- PMCID: PMC24459
- DOI: 10.1073/pnas.96.25.14464
Specific therapy regimes could lead to long-term immunological control of HIV
Abstract
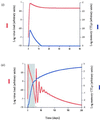
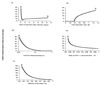
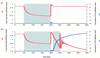
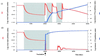
We use mathematical models to study the relationship between HIV and the immune system during the natural course of infection and in the context of different antiviral treatment regimes. The models suggest that an efficient cytotoxic T lymphocyte (CTL) memory response is required to control the virus. We define CTL memory as long-term persistence of CTL precursors in the absence of antigen. Infection and depletion of CD4(+) T helper cells interfere with CTL memory generation, resulting in persistent viral replication and disease progression. We find that antiviral drug therapy during primary infection can enable the development of CTL memory. In chronically infected patients, specific treatment schedules, either including deliberate drug holidays or antigenic boosts of the immune system, can lead to a re-establishment of CTL memory. Whether such treatment regimes would lead to long-term immunologic control deserves investigation under carefully controlled conditions.
Figures
Similar articles
-
Correlates of cytotoxic T-lymphocyte-mediated virus control: implications for immunosuppressive infections and their treatment.Philos Trans R Soc Lond B Biol Sci. 2000 Aug 29;355(1400):1059-70. doi: 10.1098/rstb.2000.0643. Philos Trans R Soc Lond B Biol Sci. 2000. PMID: 11186307 Free PMC article. Review.
-
The persistence of CTL memory.Neth J Med. 2002 Aug;60(7 Suppl):4-13; discussion 14-6. Neth J Med. 2002. PMID: 12430586 Review.
-
A new theory of cytotoxic T-lymphocyte memory: implications for HIV treatment.Philos Trans R Soc Lond B Biol Sci. 2000 Mar 29;355(1395):329-43. doi: 10.1098/rstb.2000.0570. Philos Trans R Soc Lond B Biol Sci. 2000. PMID: 10794051 Free PMC article. Review.
-
Helper-dependent vs. helper-independent CTL responses in HIV infection: implications for drug therapy and resistance.J Theor Biol. 2001 Dec 7;213(3):447-59. doi: 10.1006/jtbi.2001.2426. J Theor Biol. 2001. PMID: 11735291
-
The dual role of CD4 T helper cells in the infection dynamics of HIV and their importance for vaccination.J Theor Biol. 2002 Feb 21;214(4):633-46. doi: 10.1006/jtbi.2001.2483. J Theor Biol. 2002. PMID: 11851372
Cited by
-
Genetic therapies against HIV.Nat Biotechnol. 2007 Dec;25(12):1444-54. doi: 10.1038/nbt1367. Nat Biotechnol. 2007. PMID: 18066041 Free PMC article. Review.
-
Benchmarking tools for a priori identifiability analysis.Bioinformatics. 2023 Feb 3;39(2):btad065. doi: 10.1093/bioinformatics/btad065. Bioinformatics. 2023. PMID: 36721336 Free PMC article.
-
Modelling HIV immune response and validation with clinical data.J Biol Dyn. 2008 Oct;2(4):357-85. doi: 10.1080/17513750701813184. J Biol Dyn. 2008. PMID: 19495424 Free PMC article.
-
Heterogeneity in chronic myeloid leukaemia dynamics during imatinib treatment: role of immune responses.Proc Biol Sci. 2010 Jun 22;277(1689):1875-80. doi: 10.1098/rspb.2009.2179. Epub 2010 Feb 10. Proc Biol Sci. 2010. PMID: 20147328 Free PMC article.
-
Viral dynamics during structured treatment interruptions of chronic human immunodeficiency virus type 1 infection.J Virol. 2002 Feb;76(3):968-79. doi: 10.1128/jvi.76.3.968-979.2002. J Virol. 2002. PMID: 11773372 Free PMC article.
References
-
- Larder B A, Darby G, Richman D D. Science. 1989;243:1731–1734. - PubMed
-
- Richman D D. Trends Microbiol. 1994;2:401–407. - PubMed
-
- Condra J H, Schleif W A, Blahy O M, Gabryelski L J, Graham D J, Quintero J C, Rhodes A, Robbins H L, Roth E, Shivaprakash M, et al. Nature (London) 1995;374:569–571. - PubMed
-
- Frost S D, McLean A R. AIDS. 1994;8:323–332. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

