Assembly of the nuclear transcription and processing machinery: Cajal bodies (coiled bodies) and transcriptosomes
- PMID: 10588665
- PMCID: PMC25765
- DOI: 10.1091/mbc.10.12.4385
Assembly of the nuclear transcription and processing machinery: Cajal bodies (coiled bodies) and transcriptosomes
Abstract
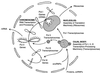
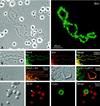
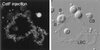
We have examined the distribution of RNA transcription and processing factors in the amphibian oocyte nucleus or germinal vesicle. RNA polymerase I (pol I), pol II, and pol III occur in the Cajal bodies (coiled bodies) along with various components required for transcription and processing of the three classes of nuclear transcripts: mRNA, rRNA, and pol III transcripts. Among these components are transcription factor IIF (TFIIF), TFIIS, splicing factors, the U7 small nuclear ribonucleoprotein particle, the stem-loop binding protein, SR proteins, cleavage and polyadenylation factors, small nucleolar RNAs, nucleolar proteins that are probably involved in pre-rRNA processing, and TFIIIA. Earlier studies and data presented here show that several of these components are first targeted to Cajal bodies when injected into the oocyte and only subsequently appear in the chromosomes or nucleoli, where transcription itself occurs. We suggest that pol I, pol II, and pol III transcription and processing components are preassembled in Cajal bodies before transport to the chromosomes and nucleoli. Most components of the pol II transcription and processing pathway that occur in Cajal bodies are also found in the many hundreds of B-snurposomes in the germinal vesicle. Electron microscopic images show that B-snurposomes consist primarily, if not exclusively, of 20- to 30-nm particles, which closely resemble the interchromatin granules described from sections of somatic nuclei. We suggest the name pol II transcriptosome for these particles to emphasize their content of factors involved in synthesis and processing of mRNA transcripts. We present a model in which pol I, pol II, and pol III transcriptosomes are assembled in the Cajal bodies before export to the nucleolus (pol I), to the B-snurposomes and eventually to the chromosomes (pol II), and directly to the chromosomes (pol III). The key feature of this model is the preassembly of the transcription and processing machinery into unitary particles. An analogy can be made between ribosomes and transcriptosomes, ribosomes being unitary particles involved in translation and transcriptosomes being unitary particles for transcription and processing of RNA.
Figures







Similar articles
-
RNA polymerase III in Cajal bodies and lampbrush chromosomes of the Xenopus oocyte nucleus.Mol Biol Cell. 2002 Oct;13(10):3466-76. doi: 10.1091/mbc.e02-05-0281. Mol Biol Cell. 2002. PMID: 12388750 Free PMC article.
-
Cajal bodies: the first 100 years.Annu Rev Cell Dev Biol. 2000;16:273-300. doi: 10.1146/annurev.cellbio.16.1.273. Annu Rev Cell Dev Biol. 2000. PMID: 11031238 Review.
-
[A role for Cajal bodies in assembly of the nuclear transcription machinery].Tsitologiia. 2003;45(10):971-5. Tsitologiia. 2003. PMID: 14989168 Review. Russian.
-
Small nuclear ribonucleoproteins and heterogeneous nuclear ribonucleoproteins in the amphibian germinal vesicle: loops, spheres, and snurposomes.J Cell Biol. 1991 May;113(3):465-83. doi: 10.1083/jcb.113.3.465. J Cell Biol. 1991. PMID: 1826687 Free PMC article.
-
Nuclear distribution of Oct-4 transcription factor in transcriptionally active and inactive mouse oocytes and its relation to RNA polymerase II and splicing factors.J Cell Biochem. 2003 Jul 1;89(4):720-32. doi: 10.1002/jcb.10545. J Cell Biochem. 2003. PMID: 12858338
Cited by
-
Cajal bodies in neurons.RNA Biol. 2017 Jun 3;14(6):712-725. doi: 10.1080/15476286.2016.1231360. Epub 2016 Sep 14. RNA Biol. 2017. PMID: 27627892 Free PMC article. Review.
-
Characterization of the nucleolar gene product, treacle, in Treacher Collins syndrome.Mol Biol Cell. 2000 Sep;11(9):3061-71. doi: 10.1091/mbc.11.9.3061. Mol Biol Cell. 2000. PMID: 10982400 Free PMC article.
-
Cell cycle-regulated phosphorylation of p220(NPAT) by cyclin E/Cdk2 in Cajal bodies promotes histone gene transcription.Genes Dev. 2000 Sep 15;14(18):2298-313. doi: 10.1101/gad.829500. Genes Dev. 2000. PMID: 10995387 Free PMC article.
-
All small nuclear RNAs (snRNAs) of the [U4/U6.U5] Tri-snRNP localize to nucleoli; Identification of the nucleolar localization element of U6 snRNA.Mol Biol Cell. 2002 Sep;13(9):3123-37. doi: 10.1091/mbc.01-12-0596. Mol Biol Cell. 2002. PMID: 12221120 Free PMC article.
-
Functional interactions with Pit-1 reorganize co-repressor complexes in the living cell nucleus.J Cell Sci. 2005 Aug 1;118(Pt 15):3277-88. doi: 10.1242/jcs.02450. Epub 2005 Jul 19. J Cell Sci. 2005. PMID: 16030140 Free PMC article.
References
-
- Bachellerie J-P, Cavaille J. Guiding ribose methylation of rRNA. Trends Biochem Sci. 1997;22:257–261. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

