Receptor-mediated endocytosis in the Caenorhabditis elegans oocyte
- PMID: 10588660
- PMCID: PMC25760
- DOI: 10.1091/mbc.10.12.4311
Receptor-mediated endocytosis in the Caenorhabditis elegans oocyte
Abstract
The Caenorhabditis elegans oocyte is a highly amenable system for forward and reverse genetic analysis of receptor-mediated endocytosis. We describe the use of transgenic strains expressing a vitellogenin::green fluorescent protein (YP170::GFP) fusion to monitor yolk endocytosis by the C. elegans oocyte in vivo. This YP170::GFP reporter was used to assay the functions of C. elegans predicted proteins homologous to vertebrate endocytosis factors using RNA-mediated interference. We show that the basic components and pathways of endocytic trafficking are conserved between C. elegans and vertebrates, and that this system can be used to test the endocytic functions of any new gene. We also used the YP170::GFP assay to identify rme (receptor-mediated endocytosis) mutants. We describe a new member of the low-density lipoprotein receptor superfamily, RME-2, identified in our screens for endocytosis defective mutants. We show that RME-2 is the C. elegans yolk receptor.
Figures
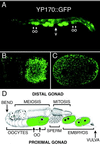



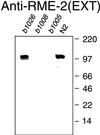
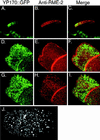
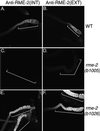
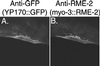
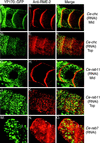
Comment in
-
An MBoC favorite: receptor-mediated endocytosis in the Caenorhabditis elegans oocyte.Mol Biol Cell. 2012 Jun;23(12):2235. doi: 10.1091/mbc.E12-02-0143. Mol Biol Cell. 2012. PMID: 22695480 Free PMC article. No abstract available.
Similar articles
-
An MBoC favorite: receptor-mediated endocytosis in the Caenorhabditis elegans oocyte.Mol Biol Cell. 2012 Jun;23(12):2235. doi: 10.1091/mbc.E12-02-0143. Mol Biol Cell. 2012. PMID: 22695480 Free PMC article. No abstract available.
-
Caenorhabditis elegans auxilin: a J-domain protein essential for clathrin-mediated endocytosis in vivo.Nat Cell Biol. 2001 Feb;3(2):215-9. doi: 10.1038/35055137. Nat Cell Biol. 2001. PMID: 11175756
-
RME-8, a conserved J-domain protein, is required for endocytosis in Caenorhabditis elegans.Mol Biol Cell. 2001 Jul;12(7):2011-21. doi: 10.1091/mbc.12.7.2011. Mol Biol Cell. 2001. PMID: 11451999 Free PMC article.
-
Deciphering endocytosis in Caenorhabditis elegans.Traffic. 2002 Jan;3(1):11-9. doi: 10.1034/j.1600-0854.2002.30103.x. Traffic. 2002. PMID: 11872138 Review.
-
Gene expression profiling of cells, tissues, and developmental stages of the nematode C. elegans.Cold Spring Harb Symp Quant Biol. 2003;68:159-69. doi: 10.1101/sqb.2003.68.159. Cold Spring Harb Symp Quant Biol. 2003. PMID: 15338614 Review. No abstract available.
Cited by
-
The CCT chaperonin and actin modulate the ER and RNA-binding protein condensation during oogenesis and maintain translational repression of maternal mRNA and oocyte quality.Mol Biol Cell. 2024 Oct 1;35(10):ar131. doi: 10.1091/mbc.E24-05-0216. Epub 2024 Aug 21. Mol Biol Cell. 2024. PMID: 39167497 Free PMC article.
-
Caenorhabditis elegans PTR/PTCHD PTR-18 promotes the clearance of extracellular hedgehog-related protein via endocytosis.PLoS Genet. 2021 Apr 19;17(4):e1009457. doi: 10.1371/journal.pgen.1009457. eCollection 2021 Apr. PLoS Genet. 2021. PMID: 33872306 Free PMC article.
-
MARC-3, a membrane-associated ubiquitin ligase, is required for fast polyspermy block in Caenorhabditis elegans.Nat Commun. 2024 Jan 26;15(1):792. doi: 10.1038/s41467-024-44928-6. Nat Commun. 2024. PMID: 38278786 Free PMC article.
-
Extracellular RNA is transported from one generation to the next in Caenorhabditis elegans.Proc Natl Acad Sci U S A. 2016 Nov 1;113(44):12496-12501. doi: 10.1073/pnas.1608959113. Epub 2016 Oct 17. Proc Natl Acad Sci U S A. 2016. PMID: 27791108 Free PMC article.
-
N-glycosylation is required for secretion and mitosis in C. elegans.PLoS One. 2013 May 14;8(5):e63687. doi: 10.1371/journal.pone.0063687. Print 2013. PLoS One. 2013. PMID: 23691084 Free PMC article.
References
-
- Bettinger JC, Lee K, Rougvie AE. Stage-specific accumulation of the terminal differentiation factor LIN-29 during Caenorhabditis elegans development. Development. 1996;122:2517–2527. - PubMed
-
- Bossinger O, Schierenberg E. Cell-cell communication in the embryo of Caenorhabditis elegans. Dev Biol. 1992;151:401–409. - PubMed
-
- Bossinger O, Schierenberg E. The use of fluorescent marker dyes for studying intercellular communication in nematode embryos. Int J Dev Biol. 1996;40:431–439. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

