Programmed cell death of embryonic motoneurons triggered through the Fas death receptor
- PMID: 10579724
- PMCID: PMC2169347
- DOI: 10.1083/jcb.147.5.1049
Programmed cell death of embryonic motoneurons triggered through the Fas death receptor
Abstract
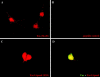
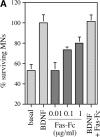
About 50% of spinal motoneurons undergo programmed cell death (PCD) after target contact, but little is known about how this process is initiated. Embryonic motoneurons coexpress the death receptor Fas and its ligand FasL at the stage at which PCD is about to begin. In the absence of trophic factors, many motoneurons die in culture within 2 d. Most (75%) of these were saved by Fas-Fc receptor body, which blocks interactions between Fas and FasL, or by the caspase-8 inhibitor tetrapeptide IETD. Therefore, activation of Fas by endogenous FasL underlies cell death induced by trophic deprivation. In the presence of neurotrophic factors, exogenous Fas activators such as soluble FasL or anti-Fas antibodies triggered PCD of 40-50% of purified motoneurons over the following 3-5 d; this treatment led to activation of caspase-3, and was blocked by IETD. Sensitivity to Fas activation is regulated: motoneurons cultured for 3 d with neurotrophic factors became completely resistant. Levels of Fas expressed by motoneurons varied little, but FasL was upregulated in the absence of neurotrophic factors. Motoneurons resistant to Fas activation expressed high levels of FLICE-inhibitory protein (FLIP), an endogenous inhibitor of caspase-8 activation. Our results suggest that Fas can act as a driving force for motoneuron PCD, and raise the possibility that active triggering of PCD may contribute to motoneuron loss during normal development and/or in pathological situations.
Figures





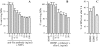
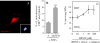
Similar articles
-
Zeta-associated protein of 70 kDa (ZAP-70), but not Syk, tyrosine kinase can mediate apoptosis of T cells through the Fas/Fas ligand, caspase-8 and caspase-3 pathways.J Immunol. 2004 Feb 1;172(3):1472-82. doi: 10.4049/jimmunol.172.3.1472. J Immunol. 2004. PMID: 14734724
-
The inhibition of apoptosis in myositis and in normal muscle cells.J Immunol. 2000 May 15;164(10):5459-65. doi: 10.4049/jimmunol.164.10.5459. J Immunol. 2000. PMID: 10799913
-
Theileria parva-transformed T cells show enhanced resistance to Fas/Fas ligand-induced apoptosis.J Immunol. 2003 Aug 1;171(3):1224-31. doi: 10.4049/jimmunol.171.3.1224. J Immunol. 2003. PMID: 12874209
-
Inhibition of fas death signals by FLIPs.Curr Opin Immunol. 1998 Oct;10(5):552-8. doi: 10.1016/s0952-7915(98)80223-9. Curr Opin Immunol. 1998. PMID: 9794838 Review.
-
Apoptosis. Molecules and mechanisms.Adv Exp Med Biol. 1999;457:217-36. Adv Exp Med Biol. 1999. PMID: 10500797 Review.
Cited by
-
Negative autoregulation by FAS mediates robust fetal erythropoiesis.PLoS Biol. 2007 Oct;5(10):e252. doi: 10.1371/journal.pbio.0050252. PLoS Biol. 2007. PMID: 17896863 Free PMC article.
-
FasL (CD95L/APO-1L) resistance of neurons mediated by phosphatidylinositol 3-kinase-Akt/protein kinase B-dependent expression of lifeguard/neuronal membrane protein 35.J Neurosci. 2005 Jul 20;25(29):6765-74. doi: 10.1523/JNEUROSCI.1700-05.2005. J Neurosci. 2005. PMID: 16033886 Free PMC article.
-
Neurotrophic factors improve motoneuron survival and function of muscle reinnervated by embryonic neurons.J Neuropathol Exp Neurol. 2009 Jul;68(7):736-46. doi: 10.1097/NEN.0b013e3181a9360f. J Neuropathol Exp Neurol. 2009. PMID: 19535998 Free PMC article.
-
Spinal muscular atrophy astrocytes exhibit abnormal calcium regulation and reduced growth factor production.Glia. 2013 Sep;61(9):1418-1428. doi: 10.1002/glia.22522. Epub 2013 Jul 10. Glia. 2013. PMID: 23839956 Free PMC article.
-
The long form of Fas apoptotic inhibitory molecule is expressed specifically in neurons and protects them against death receptor-triggered apoptosis.J Neurosci. 2007 Oct 17;27(42):11228-41. doi: 10.1523/JNEUROSCI.3462-07.2007. J Neurosci. 2007. PMID: 17942717 Free PMC article.
References
-
- Adachi M., Suematsu S., Kondo T., Ogasawara J., Tanaka T., Yoshida N., Nagata S. Targeted mutation in the Fas gene causes hyperplasia in peripheral lymphoid organs and liver. Nat. Genet. 1995;11:294–300. - PubMed
-
- Arce V., Garcès A., de Bovis B., Filippi P., Henderson C., Pettmann B., deLapeyrière O. Cardiotrophin-1 requires LIFRβ to promote survival of mouse motoneurons purified by a novel technique. J. Neurosci. Res. 1999;55:119–126. - PubMed
-
- Ashkenazi A., Dixit V.M. Death receptorssignaling and modulation. Science. 1998;281:1305–1308. - PubMed
-
- Becher B., Barker P.A., Owens T., Antel J.P. CD95-CD95Lcan the brain learn from the immune system? Trends Neurosci. 21 1998. 114 117a - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

