Phosphorylation of myosin-binding subunit (MBS) of myosin phosphatase by Rho-kinase in vivo
- PMID: 10579722
- PMCID: PMC2169354
- DOI: 10.1083/jcb.147.5.1023
Phosphorylation of myosin-binding subunit (MBS) of myosin phosphatase by Rho-kinase in vivo
Abstract
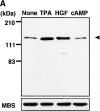
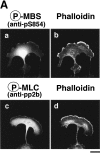
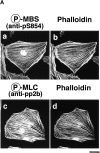
Rho-associated kinase (Rho-kinase), which is activated by the small GTPase Rho, phosphorylates myosin-binding subunit (MBS) of myosin phosphatase and thereby inactivates the phosphatase activity in vitro. Rho-kinase is thought to regulate the phosphorylation state of the substrates including myosin light chain (MLC), ERM (ezrin/radixin/moesin) family proteins and adducin by their direct phosphorylation and by the inactivation of myosin phosphatase. Here we identified the sites of phosphorylation of MBS by Rho-kinase as Thr-697, Ser-854 and several residues, and prepared antibody that specifically recognized MBS phosphorylated at Ser-854. We found by use of this antibody that the stimulation of MDCK epithelial cells with tetradecanoylphorbol-13-acetate (TPA) or hepatocyte growth factor (HGF) induced the phosphorylation of MBS at Ser-854 under the conditions in which membrane ruffling and cell migration were induced. Pretreatment of the cells with Botulinum C3 ADP-ribosyltransferase (C3), which is thought to interfere with Rho functions, or Rho-kinase inhibitors inhibited the TPA- or HGF-induced MBS phosphorylation. The TPA stimulation enhanced the immunoreactivity of phosphorylated MBS in the cytoplasm and membrane ruffling area of MDCK cells. In migrating MDCK cells, phosphorylated MBS as well as phosphorylated MLC at Ser-19 were localized in the leading edge and posterior region. Phosphorylated MBS was localized on actin stress fibers in REF52 fibroblasts. The microinjection of C3 or dominant negative Rho-kinase disrupted stress fibers and weakened the accumulation of phosphorylated MBS in REF52 cells. During cytokinesis, phosphorylated MBS, MLC and ERM family proteins accumulated at the cleavage furrow, and the phosphorylation level of MBS at Ser-854 was increased. Taken together, these results indicate that MBS is phosphorylated by Rho-kinase downstream of Rho in vivo, and suggest that myosin phosphatase and Rho-kinase spatiotemporally regulate the phosphorylation state of Rho-kinase substrates including MLC and ERM family proteins in vivo in a cooperative manner.
Figures












Similar articles
-
Association of the myosin-binding subunit of myosin phosphatase and moesin: dual regulation of moesin phosphorylation by Rho-associated kinase and myosin phosphatase.J Cell Biol. 1998 Apr 20;141(2):409-18. doi: 10.1083/jcb.141.2.409. J Cell Biol. 1998. PMID: 9548719 Free PMC article.
-
Aurora-B and Rho-kinase/ROCK, the two cleavage furrow kinases, independently regulate the progression of cytokinesis: possible existence of a novel cleavage furrow kinase phosphorylates ezrin/radixin/moesin (ERM).Genes Cells. 2005 Feb;10(2):127-37. doi: 10.1111/j.1365-2443.2005.00824.x. Genes Cells. 2005. PMID: 15676024
-
Phosphorylation of adducin by Rho-kinase plays a crucial role in cell motility.J Cell Biol. 1999 Apr 19;145(2):347-61. doi: 10.1083/jcb.145.2.347. J Cell Biol. 1999. PMID: 10209029 Free PMC article.
-
Role of myosin light chain phosphorylation in the regulation of cytokinesis.Cell Struct Funct. 2001 Dec;26(6):639-44. doi: 10.1247/csf.26.639. Cell Struct Funct. 2001. PMID: 11942620 Review.
-
The RHOad less traveled: the myosin phosphorylation-independent path from Rho kinase to cell contraction. Focus on "Rho kinase mediates serum-induced contraction in fibroblast fibers independent of myosin LC20 phosphorylation".Am J Physiol Cell Physiol. 2003 Mar;284(3):C596-8. doi: 10.1152/ajpcell.00530.2002. Am J Physiol Cell Physiol. 2003. PMID: 12556358 Review. No abstract available.
Cited by
-
Role of Rho-Associated Kinase in the Pathophysiology of Cerebral Cavernous Malformations.Neurol Genet. 2024 Jan 3;10(1):e200121. doi: 10.1212/NXG.0000000000200121. eCollection 2024 Feb. Neurol Genet. 2024. PMID: 38179414 Free PMC article. Review.
-
Novel mechanism for negatively regulating Rho-kinase (ROCK) signaling through Coronin1B protein in neuregulin 1 (NRG-1)-induced tumor cell motility.J Biol Chem. 2012 Jun 22;287(26):21836-45. doi: 10.1074/jbc.M112.346114. Epub 2012 May 4. J Biol Chem. 2012. PMID: 22563075 Free PMC article.
-
Raf-1 regulates Rho signaling and cell migration.J Cell Biol. 2005 Mar 14;168(6):955-64. doi: 10.1083/jcb.200409162. Epub 2005 Mar 7. J Cell Biol. 2005. PMID: 15753127 Free PMC article.
-
Overlapping roles of Drosophila Drak and Rok kinases in epithelial tissue morphogenesis.Mol Biol Cell. 2010 Aug 15;21(16):2869-79. doi: 10.1091/mbc.E10-04-0328. Epub 2010 Jun 23. Mol Biol Cell. 2010. PMID: 20573980 Free PMC article.
-
Citron kinase, a Rho-dependent kinase, induces di-phosphorylation of regulatory light chain of myosin II.Mol Biol Cell. 2003 May;14(5):1745-56. doi: 10.1091/mbc.e02-07-0427. Epub 2003 Feb 6. Mol Biol Cell. 2003. PMID: 12802051 Free PMC article.
References
-
- Alessi D., MacDougall L.K., Sola M.M., Ikebe M., Cohen P. The control of protein phosphatase-1 by targeting subunits. The major myosin phosphatase in avian smooth muscle is a novel form of protein phosphatase-1. Eur. J. Biochem. 1992;210:1023–1035. - PubMed
-
- Amano M., Ito M., Kimura K., Fukata Y., Chihara K., Nakano T., Matsuura Y., Kaibuchi K. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase) J. Biol. Chem. 271 1996. 20246 20249a - PubMed
-
- Amano M., Mukai H., Ono Y., Chihara K., Matsui T., Hamajima Y., Okawa K., Iwamatsu A., Kaibuchi K. Identification of a putative target for Rho as a serine-threonine kinase, PKN Science. 271 1996. 648 650b - PubMed
-
- Amano M., Chihara K., Kimura K., Fukata Y., Nakamura N., Matsuura Y., Kaibucni K. Formation of actin stress fibers and focal adhesions enhanced by Rho-kinase. Science. 1997;275:1308–1311. - PubMed
-
- Amano M., Chihara K., Nakamura N., Fukata Y., Yano T., Shibata M., Ikebe M., Kaibuchi K. Myosin II activation promotes neurite retraction during the action of Rho and Rho-kinase. Genes Cells. 1998;3:177–188. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

